Compression enhances invasive phenotype and matrix degradation of breast Cancer cells via Piezo1 activation
- PMID: 34979904
- PMCID: PMC8722159
- DOI: 10.1186/s12860-021-00401-6
Compression enhances invasive phenotype and matrix degradation of breast Cancer cells via Piezo1 activation
Abstract
Background: Uncontrolled growth in solid breast cancer generates mechanical compression that may drive the cancer cells into a more invasive phenotype, but little is known about how such compression affects the key events and corresponding regulatory mechanisms associated with invasion of breast cancer cells including cellular behaviors and matrix degradation.
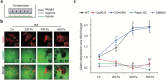
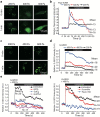
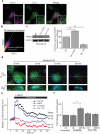
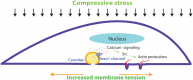
Results: Here we show that compression enhanced invasion and matrix degradation of breast cancer cells. We also identified Piezo1 as the putative mechanosensitive cellular component that transmitted compression to not only enhance the invasive phenotype, but also induce calcium influx and downstream Src signaling. Furthermore, we demonstrated that Piezo1 was mainly localized in caveolae, and both Piezo1 expression and compression-enhanced invasive phenotype of the breast cancer cells were reduced when caveolar integrity was compromised by either knocking down caveolin1 expression or depleting cholesterol content.
Conclusions: Taken together, our data indicate that mechanical compression activates Piezo1 channels to mediate enhanced breast cancer cell invasion, which involves both cellular events and matrix degradation. This may be a critical mechanotransduction pathway during breast cancer metastasis, and thus potentially a novel therapeutic target for the disease.
Keywords: Breast cancer cell; Compression; Invasion; Piezo1.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests in relation to the work described.
Figures


Similar articles
-
Compression force promotes glioblastoma progression through the Piezo1‑GDF15‑CTLA4 axis.Oncol Rep. 2025 Jan;53(1):2. doi: 10.3892/or.2024.8835. Epub 2024 Nov 8. Oncol Rep. 2025. PMID: 39513613 Free PMC article.
-
Fluid shear stress facilitates prostate cancer metastasis through Piezo1-Src-YAP axis.Life Sci. 2022 Nov 1;308:120936. doi: 10.1016/j.lfs.2022.120936. Epub 2022 Sep 6. Life Sci. 2022. PMID: 36084759
-
Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin.Elife. 2020 Mar 18;9:e52779. doi: 10.7554/eLife.52779. Elife. 2020. PMID: 32186512 Free PMC article.
-
Mechanosensing by Piezo1 and its implications for physiology and various pathologies.Biol Rev Camb Philos Soc. 2022 Apr;97(2):604-614. doi: 10.1111/brv.12814. Epub 2021 Nov 15. Biol Rev Camb Philos Soc. 2022. PMID: 34781417 Review.
-
Emerging Piezo1 signaling in inflammation and atherosclerosis; a potential therapeutic target.Int J Biol Sci. 2022 Jan 1;18(3):923-941. doi: 10.7150/ijbs.63819. eCollection 2022. Int J Biol Sci. 2022. PMID: 35173527 Free PMC article. Review.
Cited by
-
Role of extrinsic physical cues in cancer progression.BMB Rep. 2023 May;56(5):287-295. doi: 10.5483/BMBRep.2023-0031. BMB Rep. 2023. PMID: 37037673 Free PMC article. Review.
-
The impact of tumor microenvironment: unraveling the role of physical cues in breast cancer progression.Cancer Metastasis Rev. 2024 Jun;43(2):823-844. doi: 10.1007/s10555-024-10166-x. Epub 2024 Jan 19. Cancer Metastasis Rev. 2024. PMID: 38238542 Free PMC article. Review.
-
Unveiling the potential of biomechanics in pioneering innovative strategies for cancer therapy.Theranostics. 2025 Feb 10;15(7):2903-2932. doi: 10.7150/thno.108605. eCollection 2025. Theranostics. 2025. PMID: 40083943 Free PMC article. Review.
-
Adaptation to Volumetric Compression Drives an Apoptosis-Resistant and Invasive Phenotype in Liver Cancer.Cancer Res. 2025 Aug 15;85(16):3156-3175. doi: 10.1158/0008-5472.CAN-24-0859. Cancer Res. 2025. PMID: 40387600
-
The mechanopathology of the tumor microenvironment: detection techniques, molecular mechanisms and therapeutic opportunities.Front Cell Dev Biol. 2025 Mar 18;13:1564626. doi: 10.3389/fcell.2025.1564626. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40171226 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

