On-treatment blood TMB as predictors for camrelizumab plus chemotherapy in advanced lung squamous cell carcinoma: biomarker analysis of a phase III trial
- PMID: 34980131
- PMCID: PMC8722280
- DOI: 10.1186/s12943-021-01479-4
On-treatment blood TMB as predictors for camrelizumab plus chemotherapy in advanced lung squamous cell carcinoma: biomarker analysis of a phase III trial
Abstract
Background: Camrelizumab plus chemotherapy significantly prolonged progression-free survival (PFS) and overall survival (OS) compared to chemotherapy alone as first-line treatment in advanced lung squamous cell carcinoma (LUSC) in the phase III trial (CameL-sq), which has become an option of standard-of-cares for Chinese patients with advanced LUSC. However, the predictive biomarkers remain unknown.
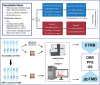
Methods: Tumor tissue samples at baseline, and peripheral blood samples at baseline (pretreatment) and after two cycles of treatment (on-treatment) were prospectively collected from 270 LUSC patients from the CameL-sq study. Blood tumor mutation burden (bTMB) and its dynamics were analyzed to explore their predictive values.
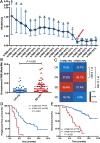
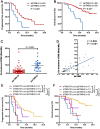
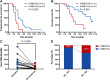
Results: Pretreatment bTMB was not associated with objective response, PFS and OS in camrelizumab or placebo plus chemotherapy groups. Low on-treatment bTMB was associated with significantly better objective response (73.8% vs 27.8%, P < 0.001), PFS (median, 9.1 vs 4.1 months; P < 0.001) and OS (median, not reached vs 8.0 months; P < 0.001) in camrelizumab plus chemotherapy group whereas it did not correlate with objective response and PFS in chemotherapy alone group. Importantly, on-treatment bTMB level could discriminate patients of initially radiological stable disease who would long-term benefit from camrelizumab plus chemotherapy (low vs high, median OS, 18.2 vs 7.8 months; P = 0.001). Combing on-treatment bTMB and its dynamics improved the ability for predicting the efficacy of camrelizumab plus chemotherapy.
Conclusion: On-treatment bTMB together with its dynamics could serve as a predictive biomarker for camrelizumab plus chemotherapy in patients with advanced LUSC.
Trial registration: ClinicalTrials.gov identifier: NCT03668496.
Keywords: PD-1; biomarker; blood tumor mutational burden; immunotherapy; lung squamous cell carcinoma.
© 2021. The Author(s).
Conflict of interest statement
CZ reported honoraria as a speaker from Roche, Lily China, Boehringer Ingelheim, Merck, Hengrui, Qilu, Sanofi, Merck Sharp & Dohme, Innovent Biologics, C-Stone, Luye Pharma, TopAlliance Biosciences, and Amoy Diagnositics; and advisor fees for Innovent Biologics, Hengrui, Qilu, and TopAlliance Biosciences. SR reported honoraria as a speaker from Boehringer Ingelheim, Lilly, Merck Sharp & Dohme, Roche, Hengrui, and Junshi, advisor fees for Roche, Merck Sharp & Dohme, and Boehringer Ingelheim and research funding from Hengrui. ZY, WS and JZ were employees of Hengrui. No other disclosures were reported.
Figures
Similar articles
-
Camrelizumab Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Squamous NSCLC (CameL-Sq): A Phase 3 Trial.J Thorac Oncol. 2022 Apr;17(4):544-557. doi: 10.1016/j.jtho.2021.11.018. Epub 2021 Dec 16. J Thorac Oncol. 2022. PMID: 34923163 Clinical Trial.
-
Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial.Lancet Respir Med. 2021 Mar;9(3):305-314. doi: 10.1016/S2213-2600(20)30365-9. Epub 2020 Dec 18. Lancet Respir Med. 2021. PMID: 33347829 Clinical Trial.
-
Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial.JAMA. 2021 Sep 14;326(10):916-925. doi: 10.1001/jama.2021.12836. JAMA. 2021. PMID: 34519801 Free PMC article. Clinical Trial.
-
The relationship between blood-based tumor mutation burden level and efficacy of PD-1/PD-L1 inhibitors in advanced non-small cell lung cancer: a systematic review and meta-analysis.BMC Cancer. 2021 Nov 13;21(1):1220. doi: 10.1186/s12885-021-08924-z. BMC Cancer. 2021. PMID: 34774004 Free PMC article.
-
Experience With Anti-PD-1 Antibody, Camrelizumab, Monotherapy for Biliary Tract Cancer Patients and Literature Review.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820979703. doi: 10.1177/1533033820979703. Technol Cancer Res Treat. 2020. PMID: 33308041 Free PMC article. Review.
Cited by
-
YAP1 as a Novel Negative Biomarker of Immune Checkpoint Inhibitors for EGFR-Mutant Non-Small-Cell Lung Cancer.Can Respir J. 2023 Jun 21;2023:4689004. doi: 10.1155/2023/4689004. eCollection 2023. Can Respir J. 2023. PMID: 37388902 Free PMC article.
-
PSMA1, a Poor Prognostic Factor, Promotes Tumor Growth in Lung Squamous Cell Carcinoma.Dis Markers. 2023 Feb 3;2023:5386635. doi: 10.1155/2023/5386635. eCollection 2023. Dis Markers. 2023. PMID: 36776923 Free PMC article.
-
Short-term dynamics of circulating tumor DNA predicting efficacy of sintilimab plus docetaxel in second-line treatment of advanced NSCLC: biomarker analysis from a single-arm, phase 2 trial.J Immunother Cancer. 2022 Dec;10(12):e004952. doi: 10.1136/jitc-2022-004952. J Immunother Cancer. 2022. PMID: 36600554 Free PMC article. Clinical Trial.
-
ctDNA-adjusted bTMB as a predictive biomarker for patients with NSCLC treated with PD-(L)1 inhibitors.BMC Med. 2022 May 5;20(1):170. doi: 10.1186/s12916-022-02360-x. BMC Med. 2022. PMID: 35509036 Free PMC article.
-
Prediction performance comparison of biomarkers for response to immune checkpoint inhibitors in advanced non-small cell lung cancer.Thorac Cancer. 2024 May;15(13):1050-1059. doi: 10.1111/1759-7714.15295. Epub 2024 Mar 25. Thorac Cancer. 2024. PMID: 38528429 Free PMC article.
References
-
- Grant MJ, Herbst RS, Goldberg SB. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat Rev Clin Oncol. 2021. 10.1038/s41571-021-00520-1. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

