High efficacy and safety of CD38 and BCMA bispecific CAR-T in relapsed or refractory multiple myeloma
- PMID: 34980210
- PMCID: PMC8722124
- DOI: 10.1186/s13046-021-02214-z
High efficacy and safety of CD38 and BCMA bispecific CAR-T in relapsed or refractory multiple myeloma
Abstract
Background: B-cell maturation antigen (BCMA) chimeric antigen receptor T (CAR-T) cell therapy has obtained promising results in relapsed or refractory multiple myeloma (R/R MM), while some patients do not response, or relapse in short term after treatment. Combining with anti-CD38 might solve the problem of targeting BCMA alone. We aimed to assess the efficacy and safety of BCMA and CD38 (BCMA-CD38) bispecific CAR-T cells in R/R MM patients.
Methods: We did a single-center, single-arm clinical study at the Second Affiliated Hospital of Yangtze University in China. Patients meeting with the inclusion criteria were administered with fludarabine and cyclophosphamide before CAR-T cells infusion. Response and adverse events were assessed after infusion. This study was registered with the Chinese Clinical Trial Registration Center (ChiCTR1900026286).
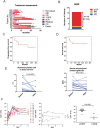
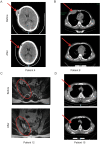
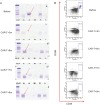
Results: First, we found BCMA-CD38 CAR-T cells exhibited enhanced killing effect on BCMA+CD38+ cells in vitro, compared to BCMA CAR-T and CD38 CAR-T cells. We further demonstrated its anti-tumor activity in vivo. Then, we enrolled 16 R/R MM patients for safety and efficacy analyses. Of the 16 evaluable patients, 14 (87.5%) respond to the treatment, including 13 stringent complete response (sCR) and one partial response (PR), while two patients did not respond. At a median follow-up of 11.5 months, of the 13 patients who achieved sCR, 76.9% (10/13) did not relapse or progress during follow-up. Relapse occurred in 3 patients (Patient 2, 3 and 4) after achieving sCR. In sum, four patients died, of which one died of hemophagocytic lymphohistiocytosis syndrome secondary to severe cytokine release syndrome (CRS) and three died of disease progression or relapse. The 1-year progression-free survival rates was 68.8%. The 1-year overall survival rate was 75.0%. Extramedullary lesions were eliminated in 62.5% (5/8) patients. The most common symptoms after CAR-T infusion were cytopenia (16, 100%), fever (10, 62.5%), fatigue (8, 50.0%) and myalgias (8, 50.0%). Twelve patients (75.0%) were observed with various grades of CRS, of which five patients (31.3%) got serious CRS (Grade ≥ 3). The CAR+ cell expansion levels were associated with the severity of CRS. Transient clonal isotype switch was observed after CAR-T infusion.
Conclusion: Our results confirm that BCMA-CD38 CAR-T cells therapy is feasible in treating R/R MM patients, with high response rate, low recurrence rate and manageable CRS, which will be a promising treatment option for R/R MM.
Trial registration: ChiCTR1900026286, registered on September 29, 2019, retrospectively registered, URL: https://www.chictr.org.cn/showproj.aspx?proj=43805.
Keywords: BCMA; CAR-T; CD38; Efficacy; Multiple myeloma; Safety.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

