Predictive and Therapeutic Implications of a Novel PLCγ1/SHP2-Driven Mechanism of Cetuximab Resistance in Metastatic Colorectal Cancer
- PMID: 34980600
- PMCID: PMC9365369
- DOI: 10.1158/1078-0432.CCR-21-1992
Predictive and Therapeutic Implications of a Novel PLCγ1/SHP2-Driven Mechanism of Cetuximab Resistance in Metastatic Colorectal Cancer
Abstract
Purpose: Cetuximab is an EGFR-targeted therapy approved for the treatment of RAS wild-type (WT) metastatic colorectal cancer (mCRC). However, about 60% of these patients show innate resistance to cetuximab. To increase cetuximab efficacy, it is crucial to successfully identify responder patients, as well as to develop new therapeutic approaches to overcome cetuximab resistance.
Experimental design: We evaluated the value of EGFR effector phospholipase C gamma 1 (PLCγ1) in predicting cetuximab responses, by analyzing progression-free survival (PFS) of a multicentric retrospective cohort of 94 treated patients with mCRC (log-rank test and Cox regression model). Furthermore, we used in vitro and zebrafish xenotransplant models to identify and target the mechanism behind PLCγ1-mediated resistance to cetuximab.
Results: In this study, levels of PLCγ1 were found increased in RAS WT tumors and were able to predict cetuximab responses in clinical samples and in vitro and in vivo models. Mechanistically, PLCγ1 expression was found to bypass cetuximab-dependent EGFR inhibition by activating ERK and AKT pathways. This novel resistance mechanism involves a noncatalytic role of PLCγ1 SH2 tandem domains in the propagation of downstream signaling via SH2-containing protein tyrosine phosphatase 2 (SHP2). Accordingly, SHP2 inhibition sensitizes PLCγ1-resistant cells to cetuximab.
Conclusions: Our discoveries reveal the potential of PLCγ1 as a predictive biomarker for cetuximab responses and suggest an alternative therapeutic approach to circumvent PLCγ1-mediated resistance to cetuximab in patients with RAS WT mCRC. In this way, this work contributes to the development of novel strategies in the medical management and treatment of patients with mCRC.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures
![Figure 1. PLCγ1 expression in colorectal cancer and its association with cetuximab responses. A, Comparison of PLCG1 mRNA expression levels in RAS WT tumors (n = 273) versus normal colonic mucosa (n = 41) from TCGA-COAD dataset. B, PLCG1 mRNA expression of paired RAS WT tumors, normal samples, and liver metastasis from AMC cohort (n = 13). C, Correlation between the levels of PLCG1 expression [RNA-seq TPM gene expression quantification from the Broad Institute Cancer Cell Line Encyclopedia (CCLE)] and cetuximab resistance (drug sensitivity measurements of GDSC from the Cancer Dependency Map Portal) in colorectal cancer RAS WT cell lines (n = 14). P value corresponds to the Spearman correlation test. Drug sensitivity was measured as AUC which corresponds to the AUC in which values of 0 correspond to complete reduction in cell viability and values of 1 correspond to no reduction in cell viability. Fitted linear regression line and its 95% CIs are indicated by the red line and shaded area, respectively. D, Cetuximab resistance of RAS WT colorectal cancer cell lines grouped by low or high PLCG1 expression based on the analysis of GDSC and CCLE data (n = 14; unpaired t test). E, Cell viability of colorectal cancer cell lines measured 72 hours after initial exposure to cetuximab (n = 4, two-way ANOVA test). F, Western blotting of EGFR and PLCγ1 downstream signaling. β-Actin was used as the loading control. G–I, Cell viability (n = 4, two-way ANOVA test), proliferation, and apoptosis rate of parental and PLCG1 overexpressing SW48 cells (pTriex-PLCG1) upon 72 hours of treatment with cetuximab (unpaired t test). J–L, Cell viability (n = 4, two-way ANOVA test), proliferation, and apoptosis rate of shControl and shPLCG1 CACO-2 cell line after 72 hours of treatment with cetuximab (unpaired t test). Results are presented as the mean ± SEM. (*, P ≤ 0.05; **, P ≤ 0.01; ****, P ≤ 0.0001).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/df51/9365369/b193daf1a2f2/1203fig1.gif)
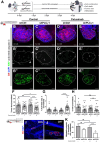

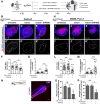
![Figure 4. Role of PLCγ1 nSH2-CSH2 domains and its interaction with SHP2 for cetuximab resistance. A and B, Western blotting of CACO-2 (shControl and shPLCG1) and SW48 (parental and pTriex-PLCG1 overexpressed) cells treated with cetuximab for 72 hr. EGFR downstream signaling, namely ERK and AKT are shown. β-actin was used as the loading control. C, Cell viability of parental SW48 and PLCG1-overexpressing cells: full length WT; constitutively active mutant - D1019K; constitutively inactive mutant - H335A; deletion of both nSH2-CSH2 tandem domains - ΔSH2; and deletions of SH3 domain - ΔSH3 (n = 3). Analysis was performed using two-way ANOVA test (****P < 0.0001). D, Western blotting of EGFR downstream signaling of SW48 parental and overexpressing PLCG1 WT and PLCG1 ΔSH2 mutant, after 72 hr of cetuximab treatment. β-Actin was used as the loading control. E, Co-immunoprecipitation of PLCγ1 with anti-Stag antibody and Western blot analysis of PLCγ1 and SHP2 of 72 hours cetuximab treated SW48 cells. F, Co-immunoprecipitation of SHP2 with anti-SHP2 antibody and Western blot analysis of PLCγ1 and SHP2 of 72 hours cetuximab treated SW48 cells. G, Proximity ligation assay of PLCγ1 and SHP2 using anti-Stag and anti-SHP2 antibodies in SW48 parental, PLCG1 WT- and ΔSH2-overexpressing cells and corresponding quantification (n = 4). H, Cell viability of SW48 parental and overexpressing PLCG1 WT and ΔSH2 mutant, treated with cetuximab and cetuximab + SHP099 for 72 hours (n = 3). I, Western blotting of EGFR downstream signaling of SW48 parental and overexpressing PLCG1 WT and ΔSH2 mutant, treated with cetuximab and cetuximab + SHP099. Results are presented as the mean ± SEM. Statistical analysis was performed using unpaired t test [not significant (ns), P > 0.05; **, P < 0.01].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/df51/9365369/cb7cc2681633/1203fig4.gif)
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
-
- Spano JP, Lagorce C, Atlan D, Milano G, Domont J, Benamouzig R, et al. Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann Oncol 2005;16:102–8. - PubMed
-
- Tabernero J, Van Cutsem E, Díaz-Rubio E, Cervantes A, Humblet Y, André T, et al. Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2007;25:5225–32. - PubMed
-
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697–705. - PubMed
-
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008;358:1160–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

