Prognostic implications of adaptive immune features in MMR-proficient colorectal liver metastases classified by histopathological growth patterns
- PMID: 34980880
- PMCID: PMC9043179
- DOI: 10.1038/s41416-021-01667-5
Prognostic implications of adaptive immune features in MMR-proficient colorectal liver metastases classified by histopathological growth patterns
Abstract
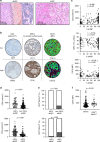
Background: After resection, colorectal cancer liver metastases (CRLM) surrounded by a desmoplastic rim carry a better prognosis than the metastases replacing the adjacent liver. However, these histopathological growth patterns (HGPs) are insufficient to guide clinical decision-making. We explored whether the adaptive immune features of HGPs could refine prognostication.
Methods: From 276 metastases resected in 176 patients classified by HGPs, tissue microarrays were used to assess intratumoral T cells (CD3), antigen presentation capacity (MHC class I) and CD73 expression producing immunosuppressive adenosine. We tested correlations between these variables and patient outcomes.
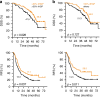
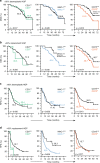
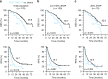
Results: The 101 (57.4%) patients with dominant desmoplastic HGP had a median recurrence-free survival (RFS) of 17.1 months compared to 13.3 months in the 75 patients (42.6%) with dominant replacement HGP (p = 0.037). In desmoplastic CRLM, high vs. low CD73 was the only prognostically informative immune parameter and was associated with a median RFS of 12.3 months compared to 26.3, respectively (p = 0.010). Only in dominant replacement CRLM, we found a subgroup (n = 23) with high intratumoral MHC-I expression but poor CD3+ T cell infiltration, a phenotype associated with a short median RFS of 7.9 months.
Conclusions: Combining the assessments of HGP and adaptive immune features in resected CRLM could help identify patients at risk of early recurrence.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
JS is a permanent member of the scientific advisory board of Surface Oncology and holds stocks of Surface Oncology. ST is receiving non-clinical research funding from Bristol-Myers Squibb, clinical research funding from GlaxoSmithKline, Iovance Biotherapeutics and Turnstone Biologics. All other authors report no conflicts of interest concerning this specific publication.
Figures
References
-
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–15. doi: 10.1016/S1470-2045(13)70447-9. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

