Probiotic supplementation in neonates with congenital gastrointestinal surgical conditions: a pilot randomised controlled trial
- PMID: 34980887
- PMCID: PMC8722408
- DOI: 10.1038/s41390-021-01884-x
Probiotic supplementation in neonates with congenital gastrointestinal surgical conditions: a pilot randomised controlled trial
Abstract
Objective: To evaluate whether probiotic supplementation attenuates gut-dysbiosis in neonates with congenital gastrointestinal surgical conditions (CGISC).
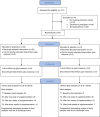
Methods: Sixty-one neonates (≥35 weeks gestation) with CGISC were randomised to receive daily supplementation with a triple-strain bifidobacterial probiotic (n = 30) or placebo (n = 31) until discharge. Stool microbiota was analysed using 16S ribosomal RNA gene sequencing on samples collected before (T1), 1 week (T2), and 2 weeks (T3) after supplementation and before discharge (T4). The primary outcome was the sum of the relative abundance of potentially pathogenic families of Clostridiaceae, Enterobacteriaceae, Enterococcaceae, Pseudomonaceae, Staphylococcaeae, Streptococcaceae, and Yersiniaceae at T3.
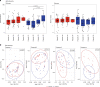
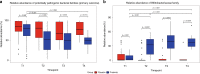
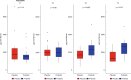
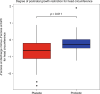
Results: The median gestational age [38 weeks (IQR: 37.1-38.9)] was similar in both groups. The probiotic group had lower rates of caesarean deliveries (40% versus 70%, p = 0.02). The relative abundance of potentially pathogenic families was lower in the probiotic group compared to placebo at T3 [(median: 50.4 (IQR: 26.6-67.6) versus 67.1 (IQR: 50.9-96.2); p = 0.04). Relative abundance of Bifidobacteriaceae was higher in the probiotic group at T3 [(median: 39.8 (IQR: 24.9-52.1) versus 0.03 (IQR 0.02-2.1); p < 0.001). Stratified analysis continued to show a higher abundance of Bifidobacteriaceae in the probiotic group, irrespective of the mode of delivery.
Conclusions: Probiotic supplementation attenuated gut dysbiosis in neonates with CGISC.
Trial registration: http://www.anzctr.org.au (ACTRN12617001401347).
Impact: Probiotic supplementation attenuates gut dysbiosis and improves stool short-chain fatty acid levels in neonates with congenital gastrointestinal surgical conditions. This is the second pilot RCT of probiotic supplementation in neonates with congenital gastrointestinal conditions. These findings will pave the way for conducting multicentre RCTs in this area.
© 2021. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Auber F, et al. Enteric nervous system impairment in gastroschisis. Eur. J. Pediatr. Surg. 2013;23:29–38. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

