Lentiviral globin gene therapy with reduced-intensity conditioning in adults with β-thalassemia: a phase 1 trial
- PMID: 34980909
- PMCID: PMC9380046
- DOI: 10.1038/s41591-021-01554-9
Lentiviral globin gene therapy with reduced-intensity conditioning in adults with β-thalassemia: a phase 1 trial
Abstract
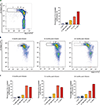
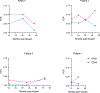
β-Thalassemias are inherited anemias that are caused by the absent or insufficient production of the β chain of hemoglobin. Here we report 6-8-year follow-up of four adult patients with transfusion-dependent β-thalassemia who were infused with autologous CD34+ cells transduced with the TNS9.3.55 lentiviral globin vector after reduced-intensity conditioning (RIC) in a phase 1 clinical trial ( NCT01639690) . Patients were monitored for insertional mutagenesis and the generation of a replication-competent lentivirus (safety and tolerability of the infusion product after RIC-primary endpoint) and engraftment of genetically modified autologous CD34+ cells, expression of the transduced β-globin gene and post-transplant transfusion requirements (efficacy-secondary endpoint). No unexpected safety issues occurred during conditioning and cell product infusion. Hematopoietic gene marking was very stable but low, reducing transfusion requirements in two patients, albeit not achieving transfusion independence. Our findings suggest that non-myeloablative conditioning can achieve durable stem cell engraftment but underscore a minimum CD34+ cell transduction requirement for effective therapy. Moderate clonal expansions were associated with integrations near cancer-related genes, suggestive of non-erythroid activity of globin vectors in stem/progenitor cells. These correlative findings highlight the necessity of cautiously monitoring patients harboring globin vectors.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing Interest Statement
The authors declare no competing financial interest. The TNS9.3.55 vector technology has been granted to Errant Gene Therapy without financial compensation.
Figures






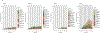
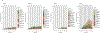
References
-
- Taher AT, Weatherall DJ & Cappellini MD Thalassaemia. Lancet 391, 155–167 (2018). - PubMed
-
- Weatherall DJ, and Clegg JB. The Thalassemia Syndrome, (Blackwell Scientific, Oxford, 1981).
-
- Orkin S, and Nathan DG. Hematology of Infancy and Childhood, (W. B. Saunders, Philadelphia, PA, 1998).
-
- Stamatoyannopoulos G The molecular basis of blood diseases, (W.B. Saunders, Philadelphia, 2001).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

