Cov19VaxKB: A Web-based Integrative COVID-19 Vaccine Knowledge Base
- PMID: 34981039
- PMCID: PMC8716025
- DOI: 10.1016/j.jvacx.2021.100139
Cov19VaxKB: A Web-based Integrative COVID-19 Vaccine Knowledge Base
Abstract
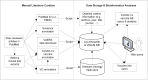
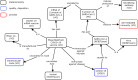
The development of SARS-CoV-2 vaccines during the COVID-19 pandemic has prompted the emergence of COVID-19 vaccine data. Timely access to COVID-19 vaccine information is crucial to researchers and public. To support more comprehensive annotation, integration, and analysis of COVID-19 vaccine information, we have developed Cov19VaxKB, a knowledge-focused COVID-19 vaccine database (http://www.violinet.org/cov19vaxkb/). Cov19VaxKB features comprehensive lists of COVID-19 vaccines, vaccine formulations, clinical trials, publications, news articles, and vaccine adverse event case reports. A web-based query interface enables comparison of product information and host responses among various vaccines. The knowledge base also includes a vaccine design tool for predicting vaccine targets and a statistical analysis tool that identifies enriched adverse events for FDA-authorized COVID-19 vaccines based on VAERS case report data. To support data exchange, Cov19VaxKB is synchronized with Vaccine Ontology and the Vaccine Investigation and Online Information Network (VIOLIN) database. The data integration and analytical features of Cov19VaxKB can facilitate vaccine research and development while also serving as a useful reference for the public.
Keywords: AE, adverse event; CDC, Centers for Disease Control and Prevention; COVID-19; COVID-19 vaccine; COVID-19, Coronavirus disease 2019; Cov19VaxKB; FDA, Food and Drug Administration; MERS-CoV, Middle Eastern Respiratory Syndrome; NCBI, National Center for Biotechnology Information; OWL, Web Ontology Language; PMID, PubMed identification number; PRR, Proportional Reporting Ratio; SARS-CoV, Severe Acute Respiratory Syndrome Coronavirus; SARS-CoV-2; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; VAERS; VAERS, Vaccine Adverse Event Reporting System; VIOLIN, Vaccine Investigation and Online Information Network; VO, Vaccine Ontology; WHO, World Health Organization; adverse event; bioinformatics; database; knowledge base; ontology; vaccine.
© 2021 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- WHO. Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio.... Accessed on July 20, 2021. 2021.
-
- WHO. COVID-19 vaccine tracker and landscape. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand.... 2021.
-
- Times TNY. Coronavirus Vaccine Tracker. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tra.... Accessed on May 26, 2021. 2021.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

