This is a preprint.
Preserved Omicron Spike specific antibody binding and Fc-recognition across COVID-19 vaccine platforms
- PMID: 34981072
- PMCID: PMC8722615
- DOI: 10.1101/2021.12.24.21268378
Preserved Omicron Spike specific antibody binding and Fc-recognition across COVID-19 vaccine platforms
Update in
-
Omicron variant Spike-specific antibody binding and Fc activity are preserved in recipients of mRNA or inactivated COVID-19 vaccines.Sci Transl Med. 2022 Apr 27;14(642):eabn9243. doi: 10.1126/scitranslmed.abn9243. Epub 2022 Apr 27. Sci Transl Med. 2022. PMID: 35289637 Free PMC article.
Abstract
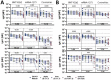
Despite the dramatic spread of Omicron globally, even among highly vaccinated populations, death rates have not increased concomitantly. These data argue that alternative immune mechanisms, beyond neutralization, may continue to confer protection against severe disease. Beyond their ability to bind and block infection, antibodies contribute to control and clearance of multiple infections via their ability to direct antiviral immunity via Fc-effector mechanisms. Thus, here we probed the ability of vaccine induced antibodies, across three COVID-19 vaccines, to drive Fc-effector activity against Omicron. Despite the significant loss of IgM, IgA and IgG binding to the Omicron Receptor Binding Domain (RBD) across BNT162b2, mRNA-1273, and CoronaVac vaccines, stable isotype binding was observed across all of these vaccines to the Omicron Spike. Compromised RBD binding IgG was accompanied by a significant loss of cross RBD-specific antibody Fcγ-receptor binding by the CoronaVac vaccine, but preservation of RBD-specific FcγR2a and Fcγ3a binding across the mRNA vaccines. Conversely, Spike-specific antibodies exhibited persistent binding to Fcγ-receptors, across all three vaccines, albeit higher binding was observed with the mRNA vaccines, marked by a selective preservation of FcγR2a and Fcγ3a binding antibodies. Thus, despite the significant to near complete loss of Omicron neutralization across several vaccine platforms against Omicron, vaccine induced Spike-specific antibodies continue to recognize the virus and recruit Fc-receptors pointing to a persistent capacity for extra-neutralizing antibodies to contribute Omicron disease attenuation.
Conflict of interest statement
Competing interests
G.A. is a founder and equity holder of Seromyx Systems, a company developing a platform technology that describes the antibody immune response. G.A. is an employee and equity holder of Leyden Labs, a company developing pandemic prevention therapeutics. G.A.’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. All other authors have declared that no conflicts of interest exist.
Figures

References
Publication types
Grants and funding
- U19 AI135995/AI/NIAID NIH HHS/United States
- HHSN272201400008C/AI/NIAID NIH HHS/United States
- R01 AI042790/AI/NIAID NIH HHS/United States
- R01 AI146785/AI/NIAID NIH HHS/United States
- UM1 AI148373/AI/NIAID NIH HHS/United States
- HHSN272201500002C/AI/NIAID NIH HHS/United States
- U19 AI135972/AI/NIAID NIH HHS/United States
- 75N93019C00052/AI/NIAID NIH HHS/United States
- P51 OD011132/OD/NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- U01 CA260476/CA/NCI NIH HHS/United States
- R37 AI080289/AI/NIAID NIH HHS/United States
- U01 AI149644/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous
