This is a preprint.
Bamlanivimab reduces nasopharyngeal SARS-CoV-2 RNA levels but not symptom duration in non-hospitalized adults with COVID-19: A Phase 2 Randomized Clinical Trial
- PMID: 34981077
- PMCID: PMC8722620
- DOI: 10.1101/2021.12.17.21268009
Bamlanivimab reduces nasopharyngeal SARS-CoV-2 RNA levels but not symptom duration in non-hospitalized adults with COVID-19: A Phase 2 Randomized Clinical Trial
Abstract
Importance: The antiviral activity and efficacy of anti-SARS-CoV-2 monoclonal antibody (mAb) therapies to accelerate recovery from COVID-19 is important to define.
Objective: To determine safety and efficacy of the mAb bamlanivimab to reduce nasopharyngeal (NP) SARS-CoV-2 RNA levels and symptom duration.
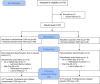
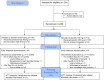
Design: ACTIV-2/A5401 is a randomized, blinded, placebo-controlled platform trial. Two dose cohorts were enrolled between August 19 and November 17, 2020 for phase 2 evaluation: in the first, participants were randomized 1:1 to bamlanivimab 7000 mg versus placebo, and in the second to bamlanivimab 700 mg versus placebo. Randomization was stratified by time from symptom onset (≤ or >5 days) and risk of progression to severe COVID-19 ("higher" vs "lower").
Setting: Multicenter trial conducted at U.S. sites.
Participants: Non-hospitalized adults ≥18 years of age with positive SARS-CoV-2 antigen or nucleic acid test within 7 days, ≤10 days of COVID-19 symptoms, and with oxygen saturation ≥92% within 48 hours prior to study entry.
Intervention: Single infusion of bamlanivimab (7000 or 700 mg) or placebo.
Main outcomes and measures: Detection of NP SARS-CoV-2 RNA at days 3, 7, 14, 21, and 28, time to improvement of all of 13 targeted COVID-19 symptoms by daily self-assessment through day 28, and grade 3 or higher treatment emergent adverse events (TEAEs) through day 28. Secondary measures included quantitative NP SARS-CoV-2 RNA, all-cause hospitalizations and deaths (composite), area under the curve of symptom scores from day 0 through day 28, plasma bamlanivimab concentrations, plasma and serum inflammatory biomarkers, and safety through week 24.
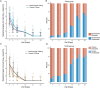
Results: Ninety-four participants were enrolled to the 7000 mg cohort and 223 to the 700 mg cohort and initiated study intervention. The proportion meeting protocol criteria for "higher" risk for COVID-19 progression was 42% and 51% for the 7000 and 700 mg cohort, respectively. Median time from symptom onset at study entry for both cohorts was 6 days. There was no difference in the proportion with undetectable NP SARS-CoV-2 RNA at any post-treatment timepoints (risk ratio compared to placebo, 0.82-1.05 for 7000 mg dose [overall p=0.88] and 0.81-1.21 for 700 mg dose [overall p=0.49]), time to symptom improvement (median of 21 vs 18.5 days, p=0.97, for 7000 mg bamlanivimab vs placebo and 24 vs 20.5 days, p=0.08, for 700 mg bamlanivimab vs placebo), or grade 3+ TEAEs with either dose compared to placebo. Median NP SARS-CoV-2 RNA levels were lower at day 3 and C-reactive protein, ferritin, and fibrinogen levels significantly reduced at days 7 and 14 for bamlanivimab 700 mg compared to placebo, with similar trends observed for bamlanivimab 7000 mg. Viral decay modeling supported more rapid decay with bamlanivimab compared to placebo.
Conclusions and relevance: Treatment with bamlanivimab 7000 mg and 700 mg was safe and compared to placebo led to more rapid reductions in NP SARS-CoV-2 RNA and inflammatory biomarkers, but did not decrease time to symptom improvement. The clinical utility of mAbs for outcomes other than hospitalizations and deaths is uncertain.
Trial registration: ClinicalTrials.gov Identifier: NCT04518410.
Figures

References
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
