Improved outcomes among newly diagnosed patients with FMS-like tyrosine kinase 3 internal tandem duplication mutated acute myeloid leukemia treated with contemporary therapy: Revisiting the European LeukemiaNet adverse risk classification
- PMID: 34981570
- PMCID: PMC8884919
- DOI: 10.1002/ajh.26451
Improved outcomes among newly diagnosed patients with FMS-like tyrosine kinase 3 internal tandem duplication mutated acute myeloid leukemia treated with contemporary therapy: Revisiting the European LeukemiaNet adverse risk classification
Abstract
Mutations in fms-like tyrosine kinase 3 (FLT3) gene are common genomic alterations in acute myeloid leukemia (AML). FLT3 internal tandem duplication mutations (FLT3-ITD) have consistently been shown to be adversely prognostic, particularly those with high allelic ratio (AR). Current AML treatment strategies, including high dose cytarabine, purine analogs, FLT3 inhibitors (FLT3i), and with or without allogeneic stem cell transplant (SCT) have been shown to improve the outcomes in patients with FLT3 mutations. We analyzed a consecutive cohort of newly diagnosed patients with AML treated at a large academic medical center from January 2012 to January 2020. A total of 1576 patients with a new diagnosis of AML were reviewed. Among these, 1438 (91%) had molecular testing for FLT3 mutations and 21% (304/1438) had an FLT3 mutation, including 17% with an FLT3-ITD mutation. We show that FLT3-ITD high AR with NPM1 wild-type have significantly improved survival compared with other European LeukemiaNet (ELN) adverse risk disease. In multivariable cox proportional hazards model of patients receiving intensive or low-intensity induction regimens, FLT3 mutations did not have prognostic significance. The use of allogeneic SCT in CR1 for patients with FLT3 mutations appears to improve survival, particularly in those with ELN adverse risk disease. Overall, this data highlights the changing prognostic impact of FLT3 mutations in a contemporary era with appropriate use of induction therapy combined with targeted agents and allogenic SCT.
© 2022 Wiley Periodicals LLC.
Figures
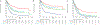
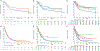
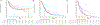
References
-
- Kiyoi H, Naoe T, Nakano Y, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93: 3074–3080. - PubMed
-
- Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98: 1752–1759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
