Increased mitochondrial fission drives the reprogramming of fatty acid metabolism in hepatocellular carcinoma cells through suppression of Sirtuin 1
- PMID: 34981667
- PMCID: PMC8753313
- DOI: 10.1002/cac2.12247
Increased mitochondrial fission drives the reprogramming of fatty acid metabolism in hepatocellular carcinoma cells through suppression of Sirtuin 1
Abstract
Background: Mitochondria are dynamic organelles that constantly change their morphology through fission and fusion processes. Recently, abnormally increased mitochondrial fission has been observed in several types of cancer. However, the functional roles of increased mitochondrial fission in lipid metabolism reprogramming in cancer cells remain unclear. This study aimed to explore the role of increased mitochondrial fission in lipid metabolism in hepatocellular carcinoma (HCC) cells.
Methods: Lipid metabolism was determined by evaluating the changes in the expressions of core lipid metabolic enzymes and intracellular lipid content. The rate of fatty acid oxidation was evaluated by [3 H]-labelled oleic acid. The mitochondrial morphology in HCC cells was evaluated by fluorescent staining. The expression of protein was determined by real-time PCR, iimmunohistochemistry and Western blotting.
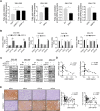
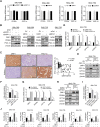
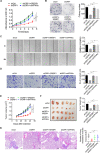
Results: Activation of mitochondrial fission significantly promoted de novo fatty acid synthesis in HCC cells through upregulating the expression of lipogenic genes fatty acid synthase (FASN), acetyl-CoA carboxylase1 (ACC1), and elongation of very long chain fatty acid protein 6 (ELOVL6), while suppressed fatty acid oxidation by downregulating carnitine palmitoyl transferase 1A (CPT1A) and acyl-CoA oxidase 1 (ACOX1). Consistently, suppressed mitochondrial fission exhibited the opposite effects. Moreover, in vitro and in vivo studies revealed that mitochondrial fission-induced lipid metabolism reprogramming significantly promoted the proliferation and metastasis of HCC cells. Mechanistically, mitochondrial fission increased the acetylation level of sterol regulatory element-binding protein 1 (SREBP1) and peroxisome proliferator-activated receptor coactivator 1 alpha (PGC-1α) by suppressing nicotinamide adenine dinucleotide (NAD+)/Sirtuin 1 (SIRT1) signaling. The elevated SREBP1 then upregulated the expression of FASN, ACC1 and ELOVL6 in HCC cells, while PGC-1α/PPARα suppressed the expression of CPT1A and ACOX1.
Conclusions: Increased mitochondrial fission plays a crucial role in the reprogramming of lipid metabolism in HCC cells, which provides strong evidence for the use of this process as a drug target in the treatment of this malignancy.
Keywords: Sirtuin 1; fatty acid oxidation; hepatocellular carcinoma; lipogenesis; metabolic reprogramming; mitochondrial fission.
© 2022 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat-sen University Cancer Center.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

