SARS-CoV-2 Delta Variant Breakthrough Infection and Onward Secondary Transmission in Household
- PMID: 34981682
- PMCID: PMC8723891
- DOI: 10.3346/jkms.2022.37.e12
SARS-CoV-2 Delta Variant Breakthrough Infection and Onward Secondary Transmission in Household
Abstract
Background: Despite the extraordinary speed of mass vaccination efforts, an outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) delta variant in a vaccinee with coronavirus disease 2019 (COVID-19) mRNA vaccine was identified in an adult day service center (ADSC) of Jeju, South Korea. The primary objective of this study was to investigate the epidemiologic features in infection-vulnerable facilities with a high vaccination rate of BNT162b2 mRNA COVID-19 vaccine. The second was to estimate the secondary transmission prevention effect of the vaccine in the household members by vaccination status.
Methods: We included all ADSC participants, staff and their household members. All COVID-19 infected cases were confirmed by reverse transcriptase polymerase chain reaction. We calculated attack rate in ADSC and the secondary attack rate (SAR) in household members by vaccination status.
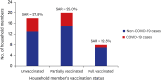
Results: Among a total of 42 participants and 16 staff, of which 96.6% were fully vaccinated with BNT162b2 mRNA COVID-19 vaccine, 12 symptomatic cases and 13 asymptomatic confirmed cases of COVID-19 were found. The attack rate was 43.1%, with 13 isolates identified as SARS-CoV-2 virus, delta variant. The SAR in unvaccinated and partially vaccinated household members were 27.8% (5/18) and 25.0% (5/20), respectively, while the SAR in fully vaccinated household members was 12.5% (1/8).
Conclusion: We describe a SARS-CoV-2 delta variant outbreak in ADSC with high vaccine coverage rate, characterized by high infection rate, high transmissibility, and low clinical severity. The outbreak proceeded to unvaccinated or partially vaccinated household members, emphasizing the need for immunizing close contacts of high-risk groups.
Keywords: COVID-19; Coronavirus; Effectiveness; SARS-CoV-2; Vaccine.
© 2022 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures


References
-
- Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373(1088):n1088. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

