Targeting memory T cell metabolism to improve immunity
- PMID: 34981777
- PMCID: PMC8718135
- DOI: 10.1172/JCI148546
Targeting memory T cell metabolism to improve immunity
Abstract
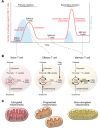
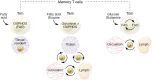
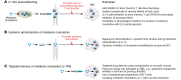
Vaccination affords protection from disease by activating pathogen-specific immune cells and facilitating the development of persistent immunologic memory toward the vaccine-specific pathogen. Current vaccine regimens are often based on the efficiency of the acute immune response, and not necessarily on the generation of memory cells, in part because the mechanisms underlying the development of efficient immune memory remain incompletely understood. This Review describes recent advances in defining memory T cell metabolism and how metabolism of these cells might be altered in patients affected by mitochondrial diseases or metabolic syndrome, who show higher susceptibility to recurrent infections and higher rates of vaccine failure. It discusses how this new understanding could add to the way we think about immunologic memory, vaccine development, and cancer immunotherapy.
Conflict of interest statement
Figures