Transcription factor BES1 interacts with HSFA1 to promote heat stress resistance of plants
- PMID: 34981847
- PMCID: PMC8804921
- DOI: 10.15252/embj.2021108664
Transcription factor BES1 interacts with HSFA1 to promote heat stress resistance of plants
Abstract
Heat stress is a major environmental stress type that can limit plant growth and development. To survive sudden temperature increases, plants utilize the heat shock response, an ancient signaling pathway. Initial results had suggested a role for brassinosteroids (BRs) in this response. Brassinosteroids are growth-promoting steroid hormones whose activity is mediated by transcription factors of the BES1/BZR1 subfamily. Here, we provide evidence that BES1 can contribute to heat stress signaling. In response to heat, BES1 is activated even in the absence of BRs and directly binds to heat shock elements (HSEs), known binding sites of heat shock transcription factors (HSFs). HSFs of the HSFA1 type can interact with BES1 and facilitate its activity in HSE binding. These findings lead us to propose an extended model of the heat stress response in plants, in which the recruitment of BES1 is a means of heat stress signaling cross-talk with a central growth regulatory pathway.
Keywords: HSFA1; brassinosteroids; heat shock factors; hormone; steroid.
© 2021 The Authors Published under the terms of the CC BY NC ND 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

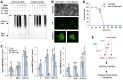
- A–C
Immunodetection of BES1‐CFP from heat‐treated plants. Plants pre‐grown at 21°C were (A) exposed for 60 min to the indicated temperatures, (B) to 45°C for the indicated period of time, or (C) to 45°C and, following sampling after 30 min (lane 2) and 60 min (lane 3), returned to 21°C for 3 h (lane 4). CBB, Coomassie Brilliant Blue stained gel.
- D
Subcellular localization of BES1‐CFP in roots of 7‐day‐old plants after heat shock. 35S:BES1‐CFP seedlings were grown at 21°C and either left untreated or exposed for 60 min to 45°C. Left: representative photos. Right: quantification of the nuclear/cytoplasmic signal. Data show the mean ± SD; n = 25. The asterisks indicate significant differences to the untreated plants by Student's t‐test (***P ≤ 0.001).
- E
Expression of the BES1‐repressed BR biosynthetic genes ROT3, DWF4, and BR6ox2 (the basic regulatory pathway is illustrated) after a heat shock of 45°C. Plants were treated as in C, samples were harvested, and qPCR analyses were performed. Data show the mean ± SD. n = 4 biological repeats, each measured in three technical replicates, normalized to UBC. The asterisks indicate significant differences compared to the control condition at 21°C by Student's t‐test (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).
- F
Heat stress resistance of bes1‐1, bes1‐2, and bes1‐D as compared to wild type. For basal resistance assays (top charts), plants were directly exposed to 45°C for the indicated time. For acquired resistance (bottom charts), plants were primed with the respective temperatures for 120 min before transfer to 45°C for 150 min. Data show the mean ± SE. n = 7. The asterisks indicate significant differences compared to wild type by Student's t‐test (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).

- A
Basal and acquired heat stress resistance of bri1‐1. For basal resistance assays (left), plants were directly exposed to 45°C for the indicated time. For acquired resistance (right), plants were primed with the respective temperatures for 120 min before transfer to 45°C for 150 min. The data are shown as means ± SE; n = 8.
- B
Immunodetection of BES1‐CFP from heat‐stressed plants, treated with epiBL or BRZ. 35S:BES1‐CFP plants were treated for 24 h with DMSO (control), 1 μM 24‐epiBL, or 5 μM BRZ and then heat‐stressed as follows: lane 1: untreated control; lane 2: 45°C for 30 min; lane 3: 45°C for 60 min; lane 4: 45°C for 60 min and recovery at 21°C for 180 min.
- C, D
Immunodetection of BES1‐CFP from heat‐treated bri1‐1 or det2‐1 plants. Left: phenotypes of 10‐day‐old plants of all lines as compared to wild‐type Col‐0 (wt). Right: Immunoblots from the lines grown and treated as in B.
- E
Basal and acquired heat stress resistance of bin2‐1. The experiment was performed as in A.
- F
Immunodetection of BES1‐CFP from heat‐treated bin2‐1 plants. The experiment was performed as in C and D.

Immunodetection of BES1‐CFP from heat‐stressed plants, treated with ABA or Flu. 35S:BES1‐CFP plants were treated for 24 h with DMSO (control), 5 μM ABA, or 10 μM Flu and heat‐stressed as follows: lane 1: untreated control; lane 2: 45°C for 30 min; lane 3: 45°C for 60 min. The non‐phosphorylated BES1 bands were quantified with ImageJ, and the obtained values are shown in blue.
Basal and acquired heat stress resistance of abi1tM. For basal resistance assays (top), plants were directly exposed to 45°C for the indicate time. For acquired resistance (bottom), plants were primed with the respective temperatures for 120 min before transfer to 45°C for 150 min. Data show the mean ± SE; n = 7. The asterisks indicate significant differences compared to wild type by Student's t‐test (*P ≤ 0.05; **P ≤ 0.01).
Immunodetection of native BES1 from heat‐stressed and epiBL‐treated abitM plants. abitM and wild‐type plants were grown on ½ MS medium either containing DMSO as a control or 1 μM epiBL and either left untreated or exposed to 45°C for 60 min. The non‐phosphorylated BES1 bands were quantified with ImageJ, and the obtained values are shown in blue.

Illustration of the promoters of HSP70.3, HSP70.4, and HSP90.1. Relevant regulatory motifs and promoter regions that were assessed in ChIP experiments (C) or in vitro DNA‐binding studies (Fig 5A) are shown with purple and gray lines, respectively.
Relative expression changes of HSP70.3, HSP70.4, and HSP90.1 in bes1‐D as compared to wild type following heat shock. 14‐day‐old plants were exposed to 45°C for 60 min, expression was analyzed by qPCR, and the results were normalized to UBC. Data show the mean ± SD. n = 3 biological replicates measured in three technical repeats. Significant differences as compared to wild type at 45°C, calculated by Student's t‐test, are shown (*P ≤ 0.05).
ChIP to determine BES1‐CFP enrichment on the promoters of HSP70.3, HSP70.4, and HSP90.1 following heat stress. 35S:BES1‐CFP and wild‐type plants grown at 21°C were either left untreated or treated with 45°C for 60 min. BES1‐CFP was immunoprecipitated with α‐GFP beads. Fragments were quantified in the precipitates with qPCR, and the ratios of samples with antibody to without antibody were calculated. TA3 (70.3p and 90.1p) or 5s rRNA (70.4p) was used for normalization. UBQ5 served as an internal control. Data show the mean ± SE. n = 3 biological replicates measured in three technical repeats. Significant differences as compared to wild type at 21°C, calculated by Student's t‐test, are shown (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).
EMSAs showing in vitro DNA interaction of recombinant BES1 and/or HSFA1 with HSE‐containing fragments of the HSP70.3 and HSP90.1 promoters. Unlabeled competitor fragments with functional HSEs (C1 +HSE) or mutated versions (C2 −HSE) were used for competition studies in 100x molar access (lanes 6 and 7).
BiFC assays showing cells co‐transformed with nYFP‐BES1 and cYFP‐HSFA1a. The fusion proteins were co‐expressed in N. benthamiana leaves, and fluorescence was visualized. Two nuclei are highlighted and shown in a 6‐fold magnification. Scale bar = 20 μm.
LUC assays in protoplasts of wild‐type, bes1‐D, or the hsfA1qM plants. LUC reporter constructs, driven by 2 kb of the HSP70.3, HSP70.4, and HSP90.1 promoters, were tested either alone (0, with empty effector plasmid) or with BES1 or HSFA1a as effectors, and LUC transactivation was analyzed. Data show the mean ± SD; n = 5. Statistically significant difference at P ≤ 0.05 of results is indicated with different letters and was determined by Student's t‐test.
Basal heat stress resistance of hsfA1qM and bes1‐DxhsfA1qM plants. Plants were directly exposed to 45°C for the indicated time. Data show the mean ± SE; n = 8.
Working model for the contribution of BES1 to heat stress signaling. BES1 is de‐phosphorylated and activated by heat stress, utilizing ABI1 and redundant ABA‐repressed PP2C phosphatases. It then interacts with HSFA1s, which are also activated by heat, for binding to HSEs in promoters of HSP70s and HSP90s genes, which induces them and increases heat stress resistance. BRs, while not needed for the activity, can promote it.

- A, B
Constitutive and BR‐responsive growth of bes1‐DxhsfA1qM seedlings. Hypocotyl elongation of 7‐day‐old dark‐grown (A) and light‐grown (B) seedlings on medium containing either 1 μM epiBL, 1 μM BRZ, or DMSO as a control (C). Left: Mean and SD of 30 plants. Significant difference at P ≤ 0.05 of results is indicated with different letters and was determined with a Student's t‐test. Right: photos of representative plants.
- C
Expression of BR‐regulated genes in bes1‐DxhsfA1qM, as compared to its parents and respective wild types analyzed by qPCRs. Data show the mean ± SD. n = 3 biological repeats, each measured in three technical replicates, normalized to UBC. Statistically significant difference at P ≤ 0.05 of results is indicated with different letters and was determined by Student's t‐test.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

