Improving the external validity of Antenatal Late Preterm Steroids trial findings
- PMID: 34981851
- PMCID: PMC9250943
- DOI: 10.1111/ppe.12856
Improving the external validity of Antenatal Late Preterm Steroids trial findings
Abstract
Background: The external validity of randomised trials can be compromised when trial participants differ from real-world populations. In the Antenatal Late Preterm Steroids (ALPS) trial of antenatal corticosteroids at late preterm ages, participants had systematically younger gestational ages than those outside the trial setting. As risk of respiratory morbidity (the primary trial outcome) is higher at younger gestations, absolute benefits of corticosteroids calculated in the trial population may overestimate real-world treatment benefits.
Objectives: To estimate the real-world absolute risk reduction and number-needed-to-treat (NNT) for antenatal corticosteroids at late preterm ages, accounting for gestational age differences between the ALPS and real-world populations.
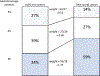
Methods: Individual participant data from the ALPS trial (which recruited 2831 women with imminent preterm birth at 34+0 to 36+5 weeks') was appended to population-based data for 15,741 women admitted for delivery between 34+0 and 36+5 weeks' from British Columbia, Canada, 2000-2013. We used logistic regression to calculate inverse odds of sampling weights for each trial participant and re-estimated treatment effects of corticosteroids on neonatal respiratory morbidity in ALPS participants, weighted to reflect the gestational age distribution of the population-based (real-world) sample.
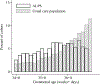
Results: The real-world absolute risk reduction was estimated to be -2.2 (95% CI -4.6, 0.0) cases of respiratory morbidity per 100, compared with -2.8 (95% CI -5.3, -0.3) in original trial data. Corresponding NNTs were 46 in the real-world setting vs 35 in the trial. Our focus on absolute measures also highlighted that the benefits of antenatal corticosteroids may be meaningfully greater at 34 weeks vs. 36 weeks (e.g., risk reductions of -3.7 vs. -1.2 per 100 respectively).
Conclusions: The absolute risk reductions and NNTs associated with antenatal corticosteroid administration at late preterm ages estimated in our study may be more appropriate for patient counselling as they better reflect the anticipated benefits of treatment when used in a real-world situation.
Keywords: Antenatal Late Preterm Steroids trial; antenatal corticosteroids; betamethasone; gestational age; neonatal respiratory morbidity.
© 2022 John Wiley & Sons Ltd.
Conflict of interest statement
Figures

Comment in
-
Point: Benchmarking can supplement transportability to answer critical questions about the effectiveness of antenatal corticosteroid administration.Paediatr Perinat Epidemiol. 2023 Jan;37(1):9-11. doi: 10.1111/ppe.12923. Epub 2022 Aug 21. Paediatr Perinat Epidemiol. 2023. PMID: 35988915 No abstract available.
-
Pledging my time: In utero exposure to acetaminophen and childhood neurodevelopment.Paediatr Perinat Epidemiol. 2023 Jul;37(5):485-486. doi: 10.1111/ppe.12985. Epub 2023 May 3. Paediatr Perinat Epidemiol. 2023. PMID: 37132479 No abstract available.
References
-
- Jordan S, Watkins A, Storey M, Allen SJ, Brooks CJ, Garaiova I, et al. Volunteer bias in recruitment, retention, and blood sample donation in a randomised controlled trial involving mothers and their children at six months and two years: a longitudinal analysis. PloS One 2013;8:e67912. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 HD053118/HD/NICHD NIH HHS/United States
- U10 HD040500/HD/NICHD NIH HHS/United States
- U10 HD068268/HD/NICHD NIH HHS/United States
- U10 HD040544/HD/NICHD NIH HHS/United States
- U01 HL098354/HL/NHLBI NIH HHS/United States
- U10 HD040485/HD/NICHD NIH HHS/United States
- U01 HL098554/HL/NHLBI NIH HHS/United States
- U10 HD034116/HD/NICHD NIH HHS/United States
- U10 HD027869/HD/NICHD NIH HHS/United States
- U10 HD027917/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- U10 HD040560/HD/NICHD NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- U10 HD040545/HD/NICHD NIH HHS/United States
- U10 HD027915/HD/NICHD NIH HHS/United States
- U10 HD068282/HD/NICHD NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- U10 HD053097/HD/NICHD NIH HHS/United States
- U10 HD068258/HD/NICHD NIH HHS/United States
- U10 HD021410/HD/NICHD NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

