Enzymatic Bromocyclization of α- and γ-Allenols by Chloroperoxidase from Curvularia inaequalis
- PMID: 34981903
- PMCID: PMC8734111
- DOI: 10.1002/open.202100236
Enzymatic Bromocyclization of α- and γ-Allenols by Chloroperoxidase from Curvularia inaequalis
Abstract
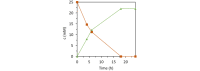
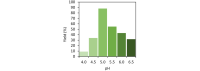
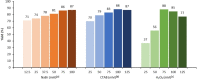
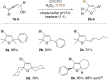
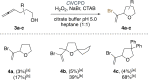
Vanadate-dependent chloroperoxidase from Curvularia inaequalis catalyzes 5-endo-trig bromocyclizations of α-allenols to produce valuable halofunctionalized furans as versatile synthetic building blocks. In contrast to other haloperoxidases, also the more challenging 5-exo-trig halocyclizations of γ-allenols succeed with this system even though the scope still remains more narrow. Benefitting from the vanadate chloroperoxidase's high resiliency towards oxidative conditions, cyclization-inducing reactive hypohalite species are generated in situ from bromide salts and hydrogen peroxide. Crucial requirements for high conversions are aqueous biphasic emulsions as reaction media, stabilized by either cationic or non-ionic surfactants.
Keywords: allenes; cyclization; halogenation; haloperoxidase; heterocycles.
© 2022 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The vanadium chloroperoxidase from the fungus, Curvularia inaequalis. Evidence for the involvement of a histidine residue in the binding of vanadate.FEBS Lett. 1993 Dec 27;336(2):239-42. doi: 10.1016/0014-5793(93)80811-8. FEBS Lett. 1993. PMID: 8262237
-
Oxidation reactions catalyzed by vanadium chloroperoxidase from Curvularia inaequalis.J Inorg Biochem. 2000 May 30;80(1-2):91-8. doi: 10.1016/s0162-0134(00)00044-1. J Inorg Biochem. 2000. PMID: 10885468
-
The chloroperoxidase from the fungus Curvularia inaequalis; a novel vanadium enzyme.Biochim Biophys Acta. 1993 Feb 13;1161(2-3):249-56. doi: 10.1016/0167-4838(93)90221-c. Biochim Biophys Acta. 1993. PMID: 8381670
-
The trigonal-bipyramidal NO4 ligand set in biologically relevant vanadium compounds and their inorganic models.J Inorg Biochem. 2008 May-Jun;102(5-6):1152-8. doi: 10.1016/j.jinorgbio.2007.12.008. Epub 2007 Dec 23. J Inorg Biochem. 2008. PMID: 18255153 Review.
-
Peritonitis due to Curvularia inaequalis in an elderly patient undergoing peritoneal dialysis and a review of six cases of peritonitis associated with other Curvularia spp.J Clin Microbiol. 2005 Aug;43(8):4288-92. doi: 10.1128/JCM.43.8.4288-4292.2005. J Clin Microbiol. 2005. PMID: 16082004 Free PMC article. Review.
Cited by
-
Halogenation in Fungi: What Do We Know and What Remains to Be Discovered?Molecules. 2022 May 14;27(10):3157. doi: 10.3390/molecules27103157. Molecules. 2022. PMID: 35630634 Free PMC article. Review.
-
Chemoenzymatic C,C-Bond Forming Cascades by Cryptic Vanadium Haloperoxidase Catalyzed Bromination.Org Lett. 2025 Jan 10;27(1):159-164. doi: 10.1021/acs.orglett.4c04108. Epub 2024 Dec 31. Org Lett. 2025. PMID: 39741038 Free PMC article.
-
Intermolecular 1,2,4-Thiadiazole Synthesis Enabled by Enzymatic Halide Recycling with Vanadium-Dependent Haloperoxidases.J Am Chem Soc. 2025 Mar 26;147(12):10698-10705. doi: 10.1021/jacs.5c01175. Epub 2025 Mar 12. J Am Chem Soc. 2025. PMID: 40071831 Free PMC article.
-
Peroxidase-induced C-N bond formation via nitroso ene and Diels-Alder reactions.Green Chem. 2023 Mar 21;25(8):3166-3174. doi: 10.1039/d2gc04827b. eCollection 2023 Apr 24. Green Chem. 2023. PMID: 37113763 Free PMC article.
-
Biocatalytic Thioketal Cleavage Enabled by Enzymatic Bromide Recycling by Vanadium-Dependent Haloperoxidases.Org Lett. 2025 Jun 6;27(22):5584-5588. doi: 10.1021/acs.orglett.5c01137. Epub 2025 May 20. Org Lett. 2025. PMID: 40393052 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

