Long-term real-world effectiveness and safety of fingolimod over 5 years in Germany
- PMID: 34982201
- PMCID: PMC9120082
- DOI: 10.1007/s00415-021-10931-w
Long-term real-world effectiveness and safety of fingolimod over 5 years in Germany
Abstract
Objective: To evaluate the 5-year real-world benefit-risk profile of fingolimod in patients with relapsing-remitting MS (RRMS) in Germany.
Methods: Post-Authorization Non-interventional German sAfety study of GilEnyA (PANGAEA) is a non-interventional real-world study to prospectively assess the effectiveness and safety of fingolimod in routine clinical practice in Germany. The follow-up period comprised 5 years. Patients were included if they had been diagnosed with RRMS and had been prescribed fingolimod as part of clinical routine. There were no exclusion criteria except the contraindications for fingolimod as defined in the European label. The effectiveness and safety analysis set comprised 4032 and 4067 RRMS patients, respectively.
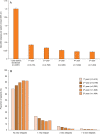
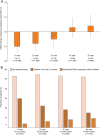
Results: At the time of the 5-year follow-up of PANGAEA, 66.57% of patients still continued fingolimod therapy. Annualized relapse rates decreased from baseline 1.5 ± 1.15 to 0.42 ± 0.734 at year 1 and 0.21 ± 0.483 at year 5, and the disability status remained stable, as demonstrated by the Expanded Disability Status Scale mean change from baseline (0.1 ± 2.51), the decrease of the Multiple Sclerosis Severity Score from 5.1 ± 2.59 at baseline to 3.9 ± 2.31 at the 60-months follow-up, and the percentage of patients with 'no change' in the Clinical Global Impression scale at the 60-months follow-up (78.11%). Adverse events (AE) occurring in 75.04% of patients were in line with the known safety profile of fingolimod and were mostly non-serious AE (33.62%) and non-serious adverse drug reactions (50.59%; serious AE 4.98%; serious ADR 10.82%).
Conclusions: PANGAEA demonstrated the sustained beneficial effectiveness and safety of fingolimod in the long-term real-world treatment of patients with RRMS.
Keywords: Effectiveness; Fingolimod; Real-world; Relapsing–remitting multiple sclerosis; Safety.
© 2022. The Author(s).
Conflict of interest statement
T.Z. has received personal compensation for participating on advisory boards, trial steering committees, and data and safety monitoring committees as well as for scientific talks and project support from Bayer HealthCare, Biogen, Celgene, Genzyme, Merck, Novartis, Roche, Sanofi, and Teva. M. L. has received speaker’s honoraria, financial research support, travel grants and consultancy fees (visiting advisory boards) from Allergan, Alnylam, Bayer, Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, Teva, Zambon. S.S. served on advisory boards and has received travel funding and speaker honoraria from Bayer Vital, Biogen, Genzyme, Merck, Novartis, Roche and Teva; S.S. has received research funding from Bayer Vital, and Merck. H.A. has received travel grants, speaker’s honoraria, and consultancy fees from Teva, Merck Serono, Genzyme, Sanofi, Novartis, Bayer, and Biogen. L. K. received compensation for serving on Scientific Advisory Boards for Genzyme, Janssen, Novartis, and Roche. She received speaker honoraria and travel support from Biogen, Genzyme, Merck Serono, Novartis, Roche, and TEVA. She receives research support from the German Ministry for Education and Research, the German Research Foundation, the IZKF Münster, IMF Münster, Biogen, Novartis, and Merck Serono. J.H. served on the scientific advisory boards of Biogen, Sanofi-Aventis, Novartis, Merck, Hoffmann La Roche received travel funding and/or speaker honoraria from Sanofi—Aventis, Merck, Bayer received research support from Octapharma, Bayer. C. L. served on the scientific advisory boards of Biogen, Celgene, Novartis, Roche and Sanofi Genzyme; received travel funding and/or speaker honoraria from Biogen, Celgene, Novartis, Roche, Sanofi Genzyme, and Teva; received research support from Biogen, Celgene, Novartis, Roche, and Sanofi Genzyme. V.E.W., B.E. and U.S.-T. are employees of Novartis.
Figures
References
-
- Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L, Group TS Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. - DOI - PubMed
-
- Kappos L, O'Connor P, Radue EW, Polman C, Hohlfeld R, Selmaj K, Ritter S, Schlosshauer R, von Rosenstiel P, Zhang-Auberson L, Francis G. Long-term effects of fingolimod in multiple sclerosis: the randomized FREEDOMS extension trial. Neurology. 2015;84:1582–1591. doi: 10.1212/wnl.0000000000001462. - DOI - PMC - PubMed
-
- Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, Vollmer T, Agius MA, Kappos L, Stites T, Li B, Cappiello L, von Rosenstiel P, Lublin FD. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:545–556. doi: 10.1016/s1474-4422(14)70049-3. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

