Heterobivalent Ligand for the Adenosine A2A-Dopamine D2 Receptor Heteromer
- PMID: 34982555
- PMCID: PMC11915710
- DOI: 10.1021/acs.jmedchem.1c01763
Heterobivalent Ligand for the Adenosine A2A-Dopamine D2 Receptor Heteromer
Abstract
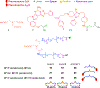
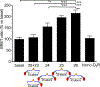
A G protein-coupled receptor heteromer that fulfills the established criteria for its existence in vivo is the complex between adenosine A2A (A2AR) and dopamine D2 (D2R) receptors. Here, we have designed and synthesized heterobivalent ligands for the A2AR-D2R heteromer with various spacer lengths. The indispensable simultaneous binding of these ligands to the two different orthosteric sites of the heteromer has been evaluated by radioligand competition-binding assays in the absence and presence of specific peptides that disrupt the formation of the heteromer, label-free dynamic mass redistribution assays in living cells, and molecular dynamic simulations. This combination of techniques has permitted us to identify compound 26 [KDB1 (A2AR) = 2.1 nM, KDB1 (D2R) = 0.13 nM], with a spacer length of 43-atoms, as a true bivalent ligand that simultaneously binds to the two different orthosteric sites. Moreover, bioluminescence resonance energy transfer experiments indicate that 26 favors the stabilization of the A2AR-D2R heteromer.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


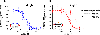



References
-
- Newman AH; Battiti FO; Bonifazi A 2016 Philip S. Portoghese Medicinal Chemistry Lectureship: Designing Bivalent or Bitopic Molecules for G-Protein Coupled Receptors. The Whole Is Greater Than the Sum of Its Parts. J. Med. Chem. 2020, 63 (5), 1779–1797. 10.1021/acs.jmedchem.9b01105. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

