Real-world Effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S Vaccines Against SARS-CoV-2 in Solid Organ and Islet Transplant Recipients
- PMID: 34982758
- PMCID: PMC8862680
- DOI: 10.1097/TP.0000000000004059
Real-world Effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S Vaccines Against SARS-CoV-2 in Solid Organ and Islet Transplant Recipients
Abstract
Background: The clinical effectiveness of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in immunosuppressed solid organ and islet transplant (SOT) recipients is unclear.
Methods: We linked 4 national registries to retrospectively identify laboratory-confirmed SARS-CoV-2 infections and deaths within 28 d in England between September 1, 2020, and August 31, 2021, comparing unvaccinated adult SOT recipients and those who had received 2 doses of ChAdOx1-S or BNT162b2 vaccine. Infection incidence rate ratios were adjusted for recipient demographics and calendar month using a negative binomial regression model, with 95% confidence intervals. Case fatality rate ratios were adjusted using a Cox proportional hazards model to generate hazard ratio (95% confidence interval).
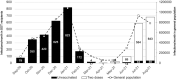
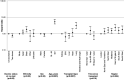
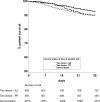
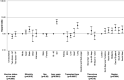
Results: On August 31, 2021, it was found that 3080 (7.1%) were unvaccinated, 1141 (2.6%) had 1 vaccine dose, and 39 260 (90.3%) had 2 vaccine doses. There were 4147 SARS-CoV-2 infections and 407 deaths (unadjusted case fatality rate 9.8%). The risk-adjusted infection incidence rate ratio was 1.29 (1.03-1.61), implying that vaccination was not associated with reduction in risk of testing positive for SARS-CoV-2 RNA. Overall, the hazard ratio for death within 28 d of SARS-CoV-2 infection was 0.80 (0.63-1.00), a 20% reduction in risk of death in vaccinated patients (P = 0.05). Two doses of ChAdOx1-S were associated with a significantly reduced risk of death (hazard ratio, 0.69; 0.52-0.92), whereas vaccination with BNT162b2 was not (0.97; 0.71-1.31).
Conclusions: Vaccination of SOT recipients confers some protection against SARS-CoV-2-related mortality, but this protection is inferior to that achieved in the general population. SOT recipients require additional protective measures, including further vaccine doses, antiviral drugs, and nonpharmaceutical interventions.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no funding or conflicts of interest.
Figures




References
-
- Danziger-Isakov L, Kumar D; AST ID Community of Practice. Vaccination of solid organ transplant candidates and recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13563. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

