Quantifying and correcting slide-to-slide variation in multiplexed immunofluorescence images
- PMID: 34983062
- PMCID: PMC8896603
- DOI: 10.1093/bioinformatics/btab877
Quantifying and correcting slide-to-slide variation in multiplexed immunofluorescence images
Abstract
Motivation: Multiplexed imaging is a nascent single-cell assay with a complex data structure susceptible to technical variability that disrupts inference. These in situ methods are valuable in understanding cell-cell interactions, but few standardized processing steps or normalization techniques of multiplexed imaging data are available.
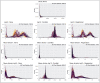
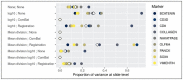
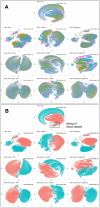
Results: We implement and compare data transformations and normalization algorithms in multiplexed imaging data. Our methods adapt the ComBat and functional data registration methods to remove slide effects in this domain, and we present an evaluation framework to compare the proposed approaches. We present clear slide-to-slide variation in the raw, unadjusted data and show that many of the proposed normalization methods reduce this variation while preserving and improving the biological signal. Furthermore, we find that dividing multiplexed imaging data by its slide mean, and the functional data registration methods, perform the best under our proposed evaluation framework. In summary, this approach provides a foundation for better data quality and evaluation criteria in multiplexed imaging.
Availability and implementation: Source code is provided at: https://github.com/statimagcoll/MultiplexedNormalization and an R package to implement these methods is available here: https://github.com/ColemanRHarris/mxnorm.
Supplementary information: Supplementary data are available at Bioinformatics online.
© The Author(s) 2022. Published by Oxford University Press.
Figures

References
-
- Bates D. et al. (2015) Fitting linear mixed-effects models using lme4. J. Stat. Softw., 67, 1–48.
-
- Becht E. et al. (2019) Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol., 37, 38–44. - PubMed
-
- Bradford J.A. et al. (2004) Fluorescence-intensity multiplexing: simultaneous seven-marker, two-color immunophenotyping using flow cytometry. Cytometry A, 61, 142–152. - PubMed

