Safety of dihydroartemisinin-piperaquine versus artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria among children in Africa: a systematic review and meta-analysis of randomized control trials
- PMID: 34983552
- PMCID: PMC8725395
- DOI: 10.1186/s12936-021-04032-2
Safety of dihydroartemisinin-piperaquine versus artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria among children in Africa: a systematic review and meta-analysis of randomized control trials
Abstract
Background: The efficacies of artemisinin based combinations have been excellent in Africa, but also comprehensive evidence regarding their safety would be important. The aim of this review was to synthesize available evidence on the safety of dihydroartemisinin-piperaquine (DHA-PQ) compared to artemether-lumefantrine (AL) for the treatment of uncomplicated Plasmodium falciparum malaria among children in Africa.
Methods: A systematic literature search was done to identify relevant articles from online databases PubMed/ MEDLINE, Embase, and Cochrane Center for Clinical Trial database (CENTRAL) for retrieving randomized control trials comparing safety of DHA-PQ and AL for treatment of uncomplicated P. falciparum malaria among children in Africa. The search was performed from August 2020 to 30 April 2021. Using Rev-Man software (V5.4.1), the extracted data from eligible studies were pooled as risk ratio (RR) with 95% confidence interval (CI).
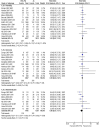
Results: In this review, 18 studies were included, which involved 10,498 participants were included. Compared to AL, DHA-PQ was associated with a slightly higher frequency of early vomiting (RR 2.26, 95% CI 1.46 to 3.50; participants = 7796; studies = 10; I2 = 0%, high quality of evidence), cough (RR 1.06, 95% CI 1.01 to 1.11; participants = 8013; studies = 13; I2 = 0%, high quality of evidence), and diarrhoea (RR 1.16, 95% CI 1.03 to 1.31; participants = 6841; studies = 11; I2 = 8%, high quality of evidence) were more frequent in DHA-PQ treatment arm.
Conclusion: From this review, it can be concluded that early vomiting, diarrhoea, and cough were common were significantly more frequent in patients who were treated with the DHA-PQ than that of AL, and both drugs are well tolerated. More studies comparing AL with DHA-PQ are needed to determine the comparative safety of these drugs.
Keywords: Adverse event; Artemether-lumefantrine; Artemisinin-based combination therapy; Children; Dihydroartemisinin-piperaquine; Meta-analysis, Africa; Pediatrics; Randomized control trial; Safety; Systematic review; Uncomplicated Plasmodium falciparum.
© 2021. The Author(s).
Conflict of interest statement
We declare that they have no competing interests.
Figures








References
-
- WHO . World Malaria Report 2019. Geneva: World Health Organization; 2019.
-
- WHO . Guidelines for treatment of malaria. 3. Geneva: World Health Organization; 2015. - PubMed
-
- World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organization; 2020.
-
- Bretscher MT, Griffin JT, Hugo P, Baker M, Ghani A, Okell L. A comparison of the duration of post-treatment protection of artemether-lumefantrine, dihydroartemisinin-piperaquine and artesunate-amodiaquine for the treatment of uncomplicated malaria. Malar J. 2014;13:19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

