Nanomedicine-enabled chemotherapy-based synergetic cancer treatments
- PMID: 34983555
- PMCID: PMC8725296
- DOI: 10.1186/s12951-021-01181-z
Nanomedicine-enabled chemotherapy-based synergetic cancer treatments
Abstract
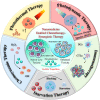
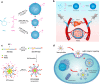
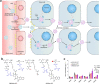
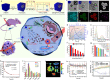
Chemotherapy remains one of the most prevailing regimens hitherto in the fight against cancer, but its development has been being suffering from various fatal side effects associated with the non-specific toxicity of common chemical drugs. Advances in biomedical application of nanomedicine have been providing alternative but promising approaches for cancer therapy, by leveraging its excellent intrinsic physicochemical properties to address these critical concerns. In particular, nanomedicine-enabled chemotherapy has been established as a safer and promising therapeutic modality, especially the recently proposed nanocatalytic medicine featuring the capabilities to generate toxic substances by initiating diverse catalytic reactions within the tumor without directly relying on highly toxic but non-selective chemotherapeutic agents. Of special note, under exogenous/endogenous stimulations, nanomedicine can serve as a versatile platform that allows additional therapeutic modalities (photothermal therapy (PTT), photodynamic therapy (PDT), chemodynamic therapy (CDT), etc.) to be seamlessly integrated with chemotherapy for efficacious synergistic treatments of tumors. Here, we comprehensively review and summarize the representative studies of multimodal synergistic cancer treatments derived from nanomedicine and nanocatalytic medicine-enabled chemotherapy in recent years, and their underlying mechanisms are also presented in detail. A number of existing challenges and further perspectives for nanomedicine-synergized chemotherapy for malignant solid tumor treatments are also highlighted for understanding this booming research area as comprehensively as possible.
Keywords: Chemotherapy; Nanomedicine; Synergisitic cancer treatments.
© 2021. The Author(s).
Conflict of interest statement
All authors declare no financial/commercial conflicts of interest.
Figures







References
-
- Chabner BA, Roberts TG. Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. - PubMed
-
- Lin H, Chen Y, Shi J. Nanoparticle-triggered in situ catalytic chemical reactions for tumour-specific therapy. Chem Soc Rev. 2018;47:1938–1958. - PubMed
-
- Matera C, Gomila AMJ, Camarero N, Libergoli M, Soler C, Gorostiza P. Photoswitchable antimetabolite for targeted photoactivated chemotherapy. J Am Chem Soc. 2018;140:15764–15773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

