Turning the tide on Alzheimer's disease: modulation of γ-secretase
- PMID: 34983641
- PMCID: PMC8725520
- DOI: 10.1186/s13578-021-00738-7
Turning the tide on Alzheimer's disease: modulation of γ-secretase
Abstract
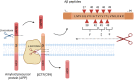
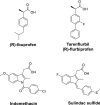
Alzheimer's disease (AD) is the most common type of neurodegenerative disorder. Amyloid-beta (Aβ) plaques are integral to the "amyloid hypothesis," which states that the accumulation of Aβ peptides triggers a cascade of pathological events leading to neurodegeneration and ultimately AD. While the FDA approved aducanumab, the first Aβ-targeted therapy, multiple safe and effective treatments will be needed to target the complex pathologies of AD. γ-Secretase is an intramembrane aspartyl protease that is critical for the generation of Aβ peptides. Activity and specificity of γ-secretase are regulated by both obligatory subunits and modulatory proteins. Due to its complex structure and function and early clinical failures with pan inhibitors, γ-secretase has been a challenging drug target for AD. γ-secretase modulators, however, have dramatically shifted the approach to targeting γ-secretase. Here we review γ-secretase and small molecule modulators, from the initial characterization of a subset of NSAIDs to the most recent clinical candidates. We also discuss the chemical biology of γ-secretase, in which small molecule probes enabled structural and functional insights into γ-secretase before the emergence of high-resolution structural studies. Finally, we discuss the recent crystal structures of γ-secretase, which have provided valuable perspectives on substrate recognition and molecular mechanisms of small molecules. We conclude that modulation of γ-secretase will be part of a new wave of AD therapeutics.
Keywords: Alzheimer’s disease; Inhibitor; Mechanism; Modulator; γ-secretase.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
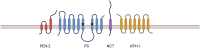
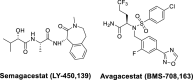
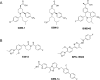
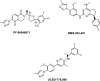
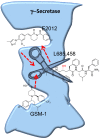
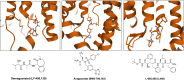
References
-
- Alzheimer's disease facts and figures. https://www.alz.org/alzheimers-dementia/facts-figures. Accessed 27 Dec 2021
-
- FDA approved treatments Alzheimer's. https://alz.org/media/documents/fda-approved-treatments-alzheimers-ts.pdf. Accessed 27 Dec 2021.
-
- Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O'Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A. The antibody aducanumab reduces Abeta plaques in Alzheimer's disease. Nature. 2016;537(7618):50–56. - PubMed
-
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120(3):885–890. - PubMed
-
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991;12(10):383–388. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

