Developmental deficits and staging of dynamics of age associated Alzheimer's disease neurodegeneration and neuronal loss in subjects with Down syndrome
- PMID: 34983655
- PMCID: PMC8728914
- DOI: 10.1186/s40478-021-01300-9
Developmental deficits and staging of dynamics of age associated Alzheimer's disease neurodegeneration and neuronal loss in subjects with Down syndrome
Abstract
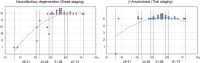
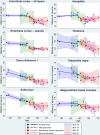
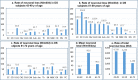
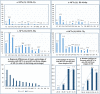
The increased life expectancy of individuals with Down syndrome (DS) is associated with increased prevalence of trisomy 21-linked early-onset Alzheimer's disease (EOAD) and dementia. The aims of this study of 14 brain regions including the entorhinal cortex, hippocampus, basal ganglia, and cerebellum in 33 adults with DS 26-72 years of age were to identify the magnitude of brain region-specific developmental neuronal deficits contributing to intellectual deficits, to apply this baseline to identification of the topography and magnitude of neurodegeneration and neuronal and volume losses caused by EOAD, and to establish age-based staging of the pattern of genetically driven neuropathology in DS. Both DS subject age and stage of dementia, themselves very strongly correlated, were strong predictors of an AD-associated decrease of the number of neurons, considered a major contributor to dementia. The DS cohort was subclassified by age as pre-AD stage, with 26-41-year-old subjects with a full spectrum of developmental deficit but with very limited incipient AD pathology, and 43-49, 51-59, and 61-72-year-old groups with predominant prevalence of mild, moderately severe, and severe dementia respectively. This multiregional study revealed a 28.1% developmental neuronal deficit in DS subjects 26-41 years of age and 11.9% AD-associated neuronal loss in DS subjects 43-49 years of age; a 28.0% maximum neuronal loss at 51-59 years of age; and a 11.0% minimum neuronal loss at 61-72 years of age. A total developmental neuronal deficit of 40.8 million neurons and AD-associated neuronal loss of 41.6 million neurons reflect a comparable magnitude of developmental neuronal deficit contributing to intellectual deficits, and AD-associated neuronal loss contributing to dementia. This highly predictable pattern of pathology indicates that successful treatment of DS subjects in the fourth decade of life may prevent AD pathology and functional decline.
Keywords: Alzheimer’s disease; Clinicopathological staging; Developmental neuronal deficits; Down syndrome; Lewy bodies; Neurofibrillary degeneration; Neuronal loss; Stereology; TDP-43 neurodegeneration; β-amyloidosis.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Amaral DG, Insausti R. Hippocampal formation. In: Paxinos G, editor. The human nervous system. San Diego, CA: Academic Press Inc; 1990. pp. 711–755.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

