Facilitation of colonic T cell immune responses is associated with an exacerbation of dextran sodium sulfate-induced colitis in mice lacking microsomal prostaglandin E synthase-1
- PMID: 34983695
- PMCID: PMC8725565
- DOI: 10.1186/s41232-021-00188-1
Facilitation of colonic T cell immune responses is associated with an exacerbation of dextran sodium sulfate-induced colitis in mice lacking microsomal prostaglandin E synthase-1
Abstract
Background: Microsomal prostaglandin E synthase-1 (mPGES-1) is a key enzyme that acts downstream of cyclooxygenase and plays a major role in inflammation by converting prostaglandin (PG) H2 to PGE2. The present study investigated the effect of genetic deletion of mPGES-1 on the development of immunologic responses to experimental colitis induced by dextran sodium sulfate (DSS), a well-established model of inflammatory bowel disease (IBD).
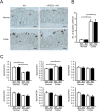
Methods: Colitis was induced in mice lacking mPGES-1 (mPGES-1-/- mice) and wild-type (WT) mice by administering DSS for 7 days. Colitis was assessed by body weight loss, diarrhea, fecal bleeding, and histological features. The colonic expression of mPGES-1 was determined by real-time PCR, western blotting, and immunohistochemistry. The impact of mPGES-1 deficiency on T cell immunity was determined by flow cytometry and T cell depletion in vivo.
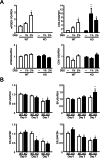
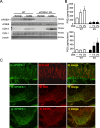
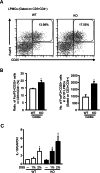
Results: After administration of DSS, mPGES-1-/- mice exhibited more severe weight loss, diarrhea, and fecal bleeding than WT mice. Histological analysis further showed significant exacerbation of colonic inflammation in mPGES-1-/- mice. In WT mice, the colonic expression of mPGES-1 was highly induced on both mRNA and protein levels and colonic PGE2 increased significantly after DSS administration. Additionally, mPGES-1 protein was localized in the colonic mucosal epithelium and infiltrated inflammatory cells in underlying connective tissues and the lamina propria. The abnormalities consistent with colitis in mPGES-1-/- mice were associated with higher expression of colonic T-helper (Th)17 and Th1 cytokines, including interleukin 17A and interferon-γ. Furthermore, lack of mPGES-1 increased the numbers of Th17 and Th1 cells in the lamina propria mononuclear cells within the colon, even though the number of suppressive regulatory T cells also increased. CD4+ T cell depletion effectively reduced symptoms of colitis as well as colonic expression of Th17 and Th1 cytokines in mPGES-1-/- mice, suggesting the requirement of CD4+ T cells in the exacerbation of DSS-induced colitis under mPGES-1 deficiency.
Conclusions: These results demonstrate that mPGES-1 is the main enzyme responsible for colonic PGE2 production and deficiency of mPGES-1 facilitates the development of colitis by affecting the development of colonic T cell-mediated immunity. mPGES-1 might therefore impact both the intestinal inflammation and T cell-mediated immunity associated with IBD.
Keywords: Colitis; Cyclooxygenase; Cytokine; Immunity; Inflammatory bowel disease; Prostaglandin E synthase; Prostaglandin E2; Th17 and Th1 response.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interest regarding the publication of this paper.
Figures






References
Grants and funding
- Integrative Research Program 2017-2018/Kitasato University Graduate School of Medical Sciences
- Student Research Program 2017-2018/Kitasato University Graduate School of Medical Sciences
- Research Program 2018/the Regenerative Medicine and Cell Design Research Facility
- Research Project 2015-1033, 2016-1011, 2017-1019/Kitasato University School of Allied Health Sciences
- Grant Number 21K06603/Japan Society for the Promotion of Science
LinkOut - more resources
Full Text Sources
Research Materials

