Metabolomic Biomarkers in Blood Samples Identify Cancers in a Mixed Population of Patients with Nonspecific Symptoms
- PMID: 34983789
- PMCID: PMC7613224
- DOI: 10.1158/1078-0432.CCR-21-2855
Metabolomic Biomarkers in Blood Samples Identify Cancers in a Mixed Population of Patients with Nonspecific Symptoms
Abstract
Purpose: Early diagnosis of cancer is critical for improving patient outcomes, but cancers may be hard to diagnose if patients present with nonspecific signs and symptoms. We have previously shown that nuclear magnetic resonance (NMR) metabolomics analysis can detect cancer in animal models and distinguish between differing metastatic disease burdens. Here, we hypothesized that biomarkers within the blood metabolome could identify cancers within a mixed population of patients referred from primary care with nonspecific symptoms, the so-called "low-risk, but not no-risk" patient group, as well as distinguishing between those with and without metastatic disease.
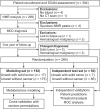
Experimental design: Patients (n = 304 comprising modeling, n = 192, and test, n = 92) were recruited from 2017 to 2018 from the Oxfordshire Suspected CANcer (SCAN) pathway, a multidisciplinary diagnostic center (MDC) referral pathway for patients with nonspecific signs and symptoms. Blood was collected and analyzed by NMR metabolomics. Orthogonal partial least squares discriminatory analysis (OPLS-DA) models separated patients, based upon diagnoses received from the MDC assessment, within 62 days of initial appointment.
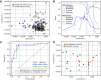
Results: Area under the ROC curve for identifying patients with solid tumors in the independent test set was 0.83 [95% confidence interval (CI): 0.72-0.95]. Maximum sensitivity and specificity were 94% (95% CI: 73-99) and 82% (95% CI: 75-87), respectively. We could also identify patients with metastatic disease in the cohort of patients with cancer with sensitivity and specificity of 94% (95% CI: 72-99) and 88% (95% CI: 53-98), respectively.
Conclusions: For a mixed group of patients referred from primary care with nonspecific signs and symptoms, NMR-based metabolomics can assist their diagnosis, and may differentiate both those with malignancies and those with and without metastatic disease. See related commentary by Van Tine and Lyssiotis, p. 1477.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures



Comment in
-
In an Era of ctDNA, Is Metabolomics the New Kid on the Block?Clin Cancer Res. 2022 Apr 14;28(8):1477-1478. doi: 10.1158/1078-0432.CCR-21-4180. Clin Cancer Res. 2022. PMID: 35140120
References
-
- Hawkes N. Cancer survival data emphasise importance of early diagnosis. BMJ 2019;364:l408. - PubMed
-
- World Health Organization. Guide to cancer early diagnosis. World Health Organization; 2017. Available from: https://www.who.int/publications/i/item/guide-to-cancer-early-diagnosis.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

