Trophic interactions with heterotrophic bacteria limit the range of Prochlorococcus
- PMID: 34983874
- PMCID: PMC8764666
- DOI: 10.1073/pnas.2110993118
Trophic interactions with heterotrophic bacteria limit the range of Prochlorococcus
Abstract
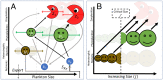
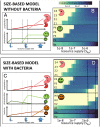
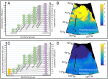
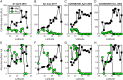
Prochlorococcus is both the smallest and numerically most abundant photosynthesizing organism on the planet. While thriving in the warm oligotrophic gyres, Prochlorococcus concentrations drop rapidly in higher-latitude regions. Transect data from the North Pacific show the collapse occurring at a wide range of temperatures and latitudes (temperature is often hypothesized to cause this shift), suggesting an ecological mechanism may be at play. An often used size-based theory of phytoplankton community structure that has been incorporated into computational models correctly predicts the dominance of Prochlorococcus in the gyres, and the relative dominance of larger cells at high latitudes. However, both theory and computational models fail to explain the poleward collapse. When heterotrophic bacteria and predators that prey nonspecifically on both Prochlorococcus and bacteria are included in the theoretical framework, the collapse of Prochlorococcus occurs with increasing nutrient supplies. The poleward collapse of Prochlorococcus populations then naturally emerges when this mechanism of "shared predation" is implemented in a complex global ecosystem model. Additionally, the theory correctly predicts trends in both the abundance and mean size of the heterotrophic bacteria. These results suggest that ecological controls need to be considered to understand the biogeography of Prochlorococcus and predict its changes under future ocean conditions. Indirect interactions within a microbial network can be essential in setting community structure.
Keywords: Prochlorococcus; biogeography; trophic interactions.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Partensky F., Garczarek L., Prochlorococcus: Advantages and limits of minimalism. Annu. Rev. Mar. Sci. 2, 305–331 (2010). - PubMed
-
- Goericke R., The structure of marine phytoplankton communities—Patterns, rules, and mechanisms. CCOFI Rep. 52, 182–197 (2011).
-
- Johnson Z. I., et al. ., Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science 311, 1737–1740 (2006). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

