Diet, lipids, and antitumor immunity
- PMID: 34983949
- PMCID: PMC8891265
- DOI: 10.1038/s41423-021-00781-x
Diet, lipids, and antitumor immunity
Abstract
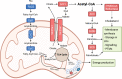
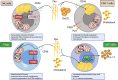
Tumour growth and dissemination is largely dependent on nutrient availability. It has recently emerged that the tumour microenvironment is rich in a diverse array of lipids that increase in abundance with tumour progression and play a role in promoting tumour growth and metastasis. Here, we describe the pro-tumorigenic roles of lipid uptake, metabolism and synthesis and detail the therapeutic potential of targeting lipid metabolism in cancer. Additionally, we highlight new insights into the distinct immunosuppressive effects of lipids in the tumour microenvironment. Lipids threaten an anti-tumour environment whereby metabolic adaptation to lipid metabolism is linked to immune dysfunction. Finally, we describe the differential effects of commondietary lipids on cancer growth which may uncover a role for specific dietary regimens in association with traditional cancer therapies. Understanding the relationship between dietary lipids, tumour, and immune cells is important in the context of obesity which may reveal a possibility to harness the diet in the treatment of cancers.
Keywords: Lipids; anti-tumour immunity; cancer; obesity; β-oxidation.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

