Factors for severe outcomes following SARS-CoV-2 infection in people with cystic fibrosis in Europe
- PMID: 34984210
- PMCID: PMC8557394
- DOI: 10.1183/23120541.00411-2021
Factors for severe outcomes following SARS-CoV-2 infection in people with cystic fibrosis in Europe
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in people with cystic fibrosis (pwCF) can lead to severe outcomes.
Methods: In this observational study, the European Cystic Fibrosis Society Patient Registry collected data on pwCF and SARS-CoV-2 infection to estimate incidence, describe clinical presentation and investigate factors associated with severe outcomes using multivariable analysis.
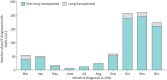
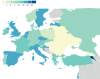
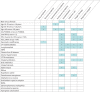
Results: Up to December 31, 2020, 26 countries reported information on 828 pwCF and SARS-CoV-2 infection. Incidence was 17.2 per 1000 pwCF (95% CI: 16.0-18.4). Median age was 24 years, 48.4% were male and 9.4% had lung transplants. SARS-CoV-2 incidence was higher in lung-transplanted (28.6; 95% CI: 22.7-35.5) versus non-lung-transplanted pwCF (16.6; 95% CI: 15.4-17.8) (p≤0.001).SARS-CoV-2 infection caused symptomatic illness in 75.7%. Factors associated with symptomatic SARS-CoV-2 infection were age >40 years, at least one F508del mutation and pancreatic insufficiency.Overall, 23.7% of pwCF were admitted to hospital, 2.5% of those to intensive care, and regretfully 11 (1.4%) died. Hospitalisation, oxygen therapy, intensive care, respiratory support and death were 2- to 6-fold more frequent in lung-transplanted versus non-lung-transplanted pwCF.Factors associated with hospitalisation and oxygen therapy were lung transplantation, cystic fibrosis-related diabetes (CFRD), moderate or severe lung disease and azithromycin use (often considered a surrogate marker for Pseudomonas aeruginosa infection and poorer lung function).
Conclusion: SARS-CoV-2 infection yielded high morbidity and hospitalisation in pwCF. PwCF with forced expiratory volume in 1 s <70% predicted, CFRD and those with lung transplants are at particular risk of more severe outcomes.
Copyright ©The authors 2021.
Conflict of interest statement
Conflict of interest: A. Jung has nothing to disclose. Conflict of interest: A. Orenti has nothing to disclose. Conflict of interest: F. Dunlevy reports support for the present manuscript from Chiesi Farmaceutici SpA. Conflict of interest: E. Aleksejeva has nothing to disclose. Conflict of interest: E. Bakkeheim has nothing to disclose. Conflict of interest: V. Bobrovnichy has nothing to disclose. Conflict of interest: S.B. Carr reports receiving speaker honoraria from Vertex and Chiesi, outside the submitted work; participation on a Data Safety Monitoring Board or Advisory Board for Vertex, Profile Pharma and Chiesi, outside the submitted work; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from UK CF Trust; PI for Pharmacovigilance study, payment to institution from Pharmaxis, outside the submitted work. Conflict of interest: C. Colombo has nothing to disclose. Conflict of interest: H. Corvol has nothing to disclose. Conflict of interest: R. Cosgrif declares outside the submitted work to be the director of the Cystic Fibrosis Trust. Conflict of interest: G. Daneau has nothing to disclose. Conflict of interest: D. Dogru has nothing to disclose. Conflict of interest: P. Drevinek reports grants or contracts from Ministry of Health, Czech Republic, outside the submitted work; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Vertex Pharmaceuticals and Actelion Pharmaceuticals, outside the submitted work; participation on a Data Safety Monitoring Board or Advisory Board for Vertex Pharmaceuticals, outside the submitted work; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from Society for Medical Microbiology, outside the submitted work. Conflict of interest: A.D. Vukic has nothing to disclose. Conflict of interest: I. Fajac reports grants or contracts from Boehringer Ingelheim, Celtaxsys, Corbus Pharmaceuticals, and Vertex Pharmaceuticals, outside the submitted work; consulting fees from Boehringer Ingelheim and Vertex Pharmaceuticals, outside the submitted work; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Vertex Pharmaceuticals and Boehringer Ingelheim, outside the submitted work; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for European Cystic Fibrosis Society. Conflict of interest: A. Fox support for the present manuscript from Chiesi Farmaceutici SpA. Conflict of interest: S. Fustik has nothing to disclose. Conflict of interest: V. Gulmans has nothing to disclose. Conflict of interest: S. Harutyunyan has nothing to disclose. Conflict of interest: E. Hatziagorou has nothing to disclose. Conflict of interest: I. Kasmi has nothing to disclose. Conflict of interest: H. Kayserová has nothing to disclose. Conflict of interest: E. Kondratyeva reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from All-Russian online school with international participation, outside the submitted work. Conflict of interest: U. Krivec has nothing to disclose. Conflict of interest: H. Makukh has nothing to disclose. Conflict of interest: K. Malakauskas has nothing to disclose. Conflict of interest: E.F. McKone reports grants or contracts from vertex, outside the submitted work; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Vertex Pharmaceuticals and Roche Pharmaceuticals, outside the submitted work; support for attending meetings and/or travel from A Menarini, outside the submitted work; participation on a Data Safety Monitoring Board or Advisory Board for Insmed and Janssen Pharmaceuticals, outside the submitted work. Conflict of interest: M. Mei-Zahav has nothing to disclose. Conflict of interest: I. de Monestrol reports grant from Vertex for an academic research study regarding gastrointestinal outcome in CF patients taking Orkambi medication, outside the submitted work; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from Swedish CF Registry and the Swedish Society of Medicine's CF working group. Conflict of interest: H.V. Olesen has nothing to disclose. Conflict of interest: R. Padoan has nothing to disclose. Conflict of interest: T. Parulava has nothing to disclose. Conflict of interest: M.D. Pastor-Vivero has nothing to disclose. Conflict of interest: L. Pereira has nothing to disclose. Conflict of interest: G. Petrova reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from TEVA Pharmaceuticals, Mylan and Alvogen, outside the submitted work. Conflict of interest: A. Pfleger has nothing to disclose. Conflict of interest: L. Pop has nothing to disclose. Conflict of interest: J.G. van Rens has nothing to disclose. Conflict of interest: M. Rodić has nothing to disclose. Conflict of interest: M. Schlesser has nothing to disclose. Conflict of interest: V. Storms has nothing to disclose. Conflict of interest: O. Turcu has nothing to disclose. Conflict of interest: L. Woźniacki has nothing to disclose. Conflict of interest: P. Yiallouros has nothing to disclose. Conflict of interest: A. Zolin has nothing to disclose. Conflict of interest: D.G. Downey has nothing to disclose. Conflict of interest: L. Naehrlich reports support for the present manuscript from Chiesi Farmaceutici SpA; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from ArticulateScience LLC, outside the submitted work; participation on a Data Safety Monitoring Board or Advisory Board for Trial Steering committee of CF Storm, outside the submitted work; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from Society for German CF Registry European CF Patient Registry, outside the submitted work; other financial or nonfinancial interests from Vertex Pharmaceuticals and Boehringer Ingelheim; Institutional fees for site participation (PI) in clinical trials from Vertex Pharmaceuticals and Boehringer Ingelheim.
Figures


References
-
- World Health Organisation . Weekly epidemiological update – 29 December 2020. www.who.int/publications/m/item/weekly-epidemiological-update---29-decem... Date last updated: 29 December 2020. Date last accessed: 23 March 2021.
LinkOut - more resources
Full Text Sources
Miscellaneous
