A phase I dose-escalation, safety/tolerability, and preliminary efficacy study of the intratumoral administration of GEN0101 in patients with advanced melanoma
- PMID: 34984539
- PMCID: PMC9293878
- DOI: 10.1007/s00262-021-03122-z
A phase I dose-escalation, safety/tolerability, and preliminary efficacy study of the intratumoral administration of GEN0101 in patients with advanced melanoma
Abstract
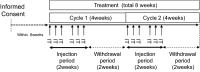
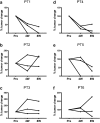
Despite recent advance in immunotherapy agents, safe new therapies that enhance the effects of immune checkpoint inhibitors are still required to develop. We previously demonstrated that hemagglutinating virus of Japan-envelope (HVJ-E) induced not only direct tumor cell death but also antitumor immunity through the activation of T and natural killer (NK) cells, thereafter, developed a manufacturing process of HVJ-E (GEN0101) for clinical use. We here performed a phase Ia clinical trial of intratumoral GEN0101 administration in six patients with stage IIIC or IV malignant melanoma. The primary aim was to evaluate the safety and tolerability of GEN0101, and the secondary aim was to examine the objective tumor response. Patients were separated into two groups (n = 3 each) and received a low dose of 30,000 and high dose of 60,000 mNAU of GEN0101. All patients completed a two-week follow-up evaluation without severe adverse events. The overall response rate was 33% (2 of 6), with 2 partial responses in the high-dose group and 2 with stable disease, and 2 with progressive disease in the low-dose group. Local complete or partial responses were observed in 11 of 18 (61%) target lesions. One patient demonstrated shrinkage of lung metastases after the treatment. The activity of NK cells and interferon-γ levels were increased in the circulation, indicating augmentation of antitumor immunity by GEN0101. This trial showed not only the safety and tolerability but also the significant antitumor effect of GEN0101, suggesting that GEN0101 might be a promising new drug for patients with advanced melanoma.
Keywords: Clinical trial; Hemagglutinating virus of Japan-envelope; Innate and adaptive immunotherapy; Melanoma; Sendai virus.
© 2021. The Author(s).
Conflict of interest statement
Toshihiro Nakajima is an employee of GenomIdea, Inc. Toshihiro Nakajima and Yasufumi Kaneda are stockholders (0.08% and 0.5%) in GenomIdea, Inc. The other authors declare no conflicts of interest.
Figures
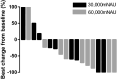


References
-
- Fujihara A, Kurooka M, Miki T, Kaneda Y. Intratumoral injection of inactivated Sendai virus particles elicits strong antitumor activity by enhancing local CXCL10 expression and systemic NK cell activation. Cancer Immunol Immunother. 2008;57:73–84. doi: 10.1007/s00262-007-0351-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

