A review on synthetic account of 1,2,4-oxadiazoles as anti-infective agents
- PMID: 34984590
- PMCID: PMC8727175
- DOI: 10.1007/s11030-021-10375-4
A review on synthetic account of 1,2,4-oxadiazoles as anti-infective agents
Abstract
Most of the currently marketed drugs consist of heterocyclic scaffolds containing nitrogen and or oxygen as heteroatoms in their structures. Several research groups have synthesized diversely substituted 1,2,4-oxadiazoles as anti-infective agents having anti-bacterial, anti-viral, anti-leishmanial, etc. activities. For the first time, the present review article will provide the coverage of synthetic account of 1,2,4-oxadiazoles as anti-infective agents along with their potential for SAR, activity potential, promising target for mode of action. The efforts have been made to provide the chemical intuitions to the reader to design new chemical entity with potential of anti-infective activity. This review will mark the impact as the valuable, comprehensive and pioneered work along with the library of synthetic strategies for the organic and medicinal chemists for further refinement of 1,2,4-oxadiazole as anti-infective agents.
Keywords: 1,2,4-Oxadiazoles; Anti-bacterial; Anti-infective; Anti-malarial; Synthesis.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


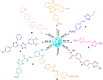
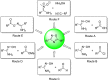
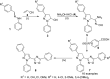

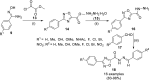
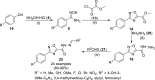
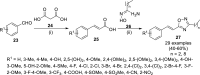

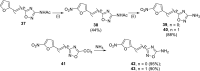
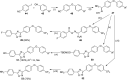

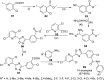
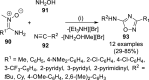
References
-
- Global Tuberculosis Report 2021. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa.... Accessed 27 Oct 2021
-
- Malaria. https://www.who.int/news-room/fact-sheets/detail/malaria. Accessed 15 Oct 2021
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

