Dendrimer-2PMPA Delays Muscle Function Loss and Denervation in a Murine Model of Amyotrophic Lateral Sclerosis
- PMID: 34984651
- PMCID: PMC9130402
- DOI: 10.1007/s13311-021-01159-7
Dendrimer-2PMPA Delays Muscle Function Loss and Denervation in a Murine Model of Amyotrophic Lateral Sclerosis
Abstract
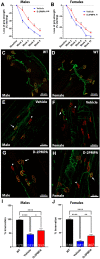
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease where muscle weakness and neuromuscular junction (NMJ) denervation precede motor neuron cell death. Although acetylcholine is the canonical neurotransmitter at the mammalian NMJ synapse, glutamate has recently been identified as a critical neurotransmitter for NMJ development and maintenance. One source of glutamate is through the catabolism of N-acetyl-aspartyl-glutamate (NAAG), which is found in mM concentrations in mammalian motoneurons, where it is released upon stimulation and hydrolyzed to glutamate by the glial enzyme glutamate carboxypeptidase II (GCPII). Using the SOD1G93A model of ALS, we found an almost fourfold elevation of GCPII enzymatic activity in SOD1G93A versus WT muscle and a robust increase in GCPII expression which was specifically associated with activated macrophages infiltrating the muscle. 2-(Phosphonomethyl)pentanedioic acid (2PMPA) is a potent GCPII inhibitor which robustly blocks glutamate release from NAAG but is highly polar with limited tissue penetration. To improve this, we covalently attached 2PMPA to a hydroxyl polyamidoamine (PAMAM-G4-OH) dendrimer delivery system (D-2PMPA) which is known to target activated macrophages in affected tissues. Systemic D-2PMPA therapy (20 mg/kg 2PMPA equivalent; IP 2 × /week) was found to localize in muscle macrophages in SOD1G93A mice and completely normalize the enhanced GCPII activity. Although no changes in body weight or survival were observed, D-2PMPA significantly improved grip strength and inhibited the loss of NMJ innervation in the gastrocnemius muscles. Our finding that inhibiting elevated GCPII activity in SOD1G93A muscle can prolong muscle function and delay NMJ denervation may have early therapeutic implications for ALS patients.
Keywords: Amyotrophic lateral sclerosis; Dendrimer; Glutamate; Glutamate carboxypeptidase II (GCPII); N-acetyl-aspartyl-glutamate (NAAG); NMDA; Neuroinflammation; Neuromuscular junction (NJM); mGluR3.
© 2021. The American Society for Experimental NeuroTherapeutics, Inc.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

