Epigenetic modifications in pancreas development, diabetes, and therapeutics
- PMID: 34984701
- PMCID: PMC9306699
- DOI: 10.1002/med.21878
Epigenetic modifications in pancreas development, diabetes, and therapeutics
Abstract
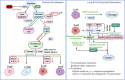
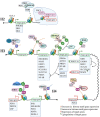
A recent International Diabetes Federation report suggests that more than 463 million people between 20 and 79 years have diabetes. Of the 20 million women affected by hyperglycemia during pregnancy, 84% have gestational diabetes. In addition, more than 1.1 million children or adolescents are affected by type 1 diabetes. Factors contributing to the increase in diabetes prevalence are complex and include contributions from genetic, environmental, and epigenetic factors. However, molecular regulatory mechanisms influencing the progression of an individual towards increased susceptibility to metabolic diseases such as diabetes are not fully understood. Recent studies suggest that the pathogenesis of diabetes involves epigenetic changes, resulting in a persistently dysregulated metabolic phenotype. This review summarizes the role of epigenetic mechanisms, mainly DNA methylation and histone modifications, in the development of the pancreas, their contribution to the development of diabetes, and the potential employment of epigenetic modulators in diabetes treatment.
Keywords: DNA methylation; epigenetic modulators; gestational diabetes; histone modifications; pancreas; type 1 diabetes; type 2 diabetes.
© 2021 The Authors. Medicinal Research Reviews published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures


References
-
- Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90‐year perspective. Postgrad Med J. 2016;92(1084):63‐69. - PubMed
-
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389(6649):349‐352. - PubMed
-
- Peterson CL, Laniel MA. Histones and histone modifications. Current Biology: CB. 2004;14(14):R546‐R551. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

