Synthesis of Polyoxygenated Tropolones and their Antiviral Activity against Hepatitis B Virus and Herpes Simplex Virus-1
- PMID: 34984767
- PMCID: PMC8858858
- DOI: 10.1002/chem.202104112
Synthesis of Polyoxygenated Tropolones and their Antiviral Activity against Hepatitis B Virus and Herpes Simplex Virus-1
Abstract
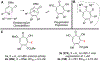
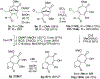
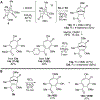
Polyoxygenated tropolones possess a broad range of biological activity, and as a result are promising lead structures or fragments for drug development. However, structure-function studies and subsequent optimization have been challenging, in part due to the limited number of readily available tropolones and the obstacles to their synthesis. Oxidopyrylium [5+2] cycloaddition can effectively generate a diverse array of seven-membered ring carbocycles, and as a result can provide a highly general strategy for tropolone synthesis. Here, we describe the use of 3-hydroxy-4-pyrone-based oxidopyrylium cycloaddition chemistry in the synthesis of functionalized 3,7-dimethoxytropolones, 3,7-dihydroxytropolones, and isomeric 3-hydroxy-7-methoxytropolones through complementary benzyl alcohol-incorporating procedures. The antiviral activity of these molecules against herpes simplex virus-1 and hepatitis B virus is also described, highlighting the value of this approach and providing new structure-function insights relevant to their antiviral activity.
Keywords: hepatitis B antivirals; herpes simplex virus antivirals; oxidopyrylium cycloaddition; structure-function analysis; tropolones.
© 2022 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interest Statement
LAM, JET, and RPM are inventors on patents describing the antiviral activity of tropolones against herpes simplex virus-1 and −2 and hepatitis B virus.
Figures




Similar articles
-
Synthesis and biological assessment of 3,7-dihydroxytropolones.Org Biomol Chem. 2017 Dec 19;16(1):62-69. doi: 10.1039/c7ob02453c. Org Biomol Chem. 2017. PMID: 29098212 Free PMC article.
-
Tropolones and Thailandepsin B as Lead-like Natural Compounds in the Development of Potent and Selective Histone Deacetylase Inhibitors.Curr Drug Targets. 2023;24(9):698-717. doi: 10.2174/1389450124666230707144251. Curr Drug Targets. 2023. PMID: 37424350
-
Discovery and Development of a Three-Component Oxidopyrylium [5 + 2] Cycloaddition.J Org Chem. 2016 May 6;81(9):3744-51. doi: 10.1021/acs.joc.6b00394. Epub 2016 Apr 8. J Org Chem. 2016. PMID: 27018974 Free PMC article.
-
Herpes simplex virus type-1: replication, latency, reactivation and its antiviral targets.Antivir Ther. 2016;21(4):277-86. doi: 10.3851/IMP3018. Epub 2016 Jan 4. Antivir Ther. 2016. PMID: 26726828 Review.
-
Chemical mechanisms involved during the biosynthesis of tropolones.Curr Opin Chem Biol. 2013 Aug;17(4):532-6. doi: 10.1016/j.cbpa.2013.06.029. Epub 2013 Jul 16. Curr Opin Chem Biol. 2013. PMID: 23870699 Review.
Cited by
-
Lactam-fused tropolones: a new tunable, environmentally sensitive fluorophore class.Org Biomol Chem. 2023 Oct 11;21(39):7900-7907. doi: 10.1039/d3ob01263h. Org Biomol Chem. 2023. PMID: 37750360 Free PMC article.
-
In vitro evaluation of tropolone absorption, metabolism, and clearance.Antiviral Res. 2023 Dec;220:105762. doi: 10.1016/j.antiviral.2023.105762. Epub 2023 Nov 22. Antiviral Res. 2023. PMID: 37996012 Free PMC article.
-
Carbocycloaddition Strategies for Troponoid Synthesis.Tetrahedron. 2023 Jan 9;130:133175. doi: 10.1016/j.tet.2022.133175. Epub 2022 Dec 1. Tetrahedron. 2023. PMID: 36777111 Free PMC article.
References
-
- For select examples, see: Iwatsuki M, Takada S, Mori M, Ishiyama A, Namatame M, Otoguro K, Nishihara-Tsukashima A, Nonaka K, Masuma R, Otoguro O, Shiomi K, Omura S, J. Antibiot 2011, 64, 183–188.; - PubMed
- Sugawara K, Ohbayashi M, Shimizu K, Hatori M, Kamei H, Konishi M, Oki T, Kawaguchi H, J. Antibiot 1988, 41, 862–868.; - PubMed
- Credille CV, Dick BL, Morrison CN, Stokes RW, Adamek RN, Wu NC, Wilson IA, Cohen SM, S. M. J. Med. Chem 2018, 61, 10206–10217.; - PMC - PubMed
- For review, see: Meck C, D'Erasmo MP, Hirsch DR, Murelli RP, Med. Chem. Commun 2014, 5, 842–852. - PMC - PubMed
-
- For some examples of synthetic chemistry-driven SAR, see: Piettre SR, Andre C, Chanal MC, Ducep JB, Lesur B, Piriou F, Raboisson P, Rondeau JM, Schelcher C, Zimmermann P, Ganzhorn AJ, J. Med. Chem 1997, 40, 4208–4221.; - PubMed
- Didierjean J, Isel C, Querre F, Mouscadet J-F, Aubertin A-M, Valnot J-Y, Piettre SR, Marquet R, Antimicrob. Agents Chemother 2005, 49, 4884–4894.; - PMC - PubMed
- Chung S, Himmel D, Jiang J-K, Wojtak K, Bauman JD, Rausch JW, Wilson JA, Beutler JA, Thomas CJ, Arnold E, Le Grice SFJ, J. Med. Chem 2011, 54, 4462–4473.; - PMC - PubMed
- Sennari G, Saito R, Hirose T, Iwatsuki M, Ishayama A, Hokari R, Otoguro K, Omura S, Sunazuka T, Sci. Rep 2017, 7, 7259.; - PMC - PubMed
- Agyemang NB, Kukla CR, Edwards TC, Li Q, Langen MK, Schaal A, Franson AD, Gazquez Casals A, Donald KA, Yu AJ, Donlin MJ, Morrison LA, Tavis JE, Murelli RP, RSC Advances, 2019, 9, 34227–34234. - PMC - PubMed
- Saito R, Sennari G, Nakajima A, Kiminishi A, Iwatsuki M, Ishiyawa A, Hokari R, Hirose T, Sunazuka T, Chem. Pharm. Bull 2021, 69, 564–572. - PubMed
-
- Murelli RP, D'Erasmo MP, Hirsch DR, Meck C, Masaoka T, Wilson JA, Zhang B, Pal RK, Gallicchio E, Beutler JA, Le Grice SFJ, Med. Chem. Commun 2016, 9, 1783–1788.; - PMC - PubMed
- Zhang B, D'Erasmo MP, Murelli RP, Gallicchio E, ACS Omega, 2016, 1, 435–447.; - PMC - PubMed
- For seminal studies, see Budihas SR, Gorshkova I, Gaidamakov S, Wamiru A, Bona MK, Parniak MA, Crouch RJ, McMahon JB, Beutler JA, Le Grice SFJ, Nucleic Acids Res. 2005, 33, 1249–1256. - PMC - PubMed
-
- Lu G, Lomonosova E, Cheng X, Moran EA, Meyers MJ, Le Grice SFJ, Thomas CJ, Jiang J-K, Meck C, Hirsch DR, R. D; D'Erasmo MP, Suyabatmaz DM, Murelli RP, Tavis JE, Antimicrob. Agents Chemother 2015, 59, 1070–1079.; - PMC - PubMed
- Lomonosova E, Daw J, J.; Garimallaprabhakaran AK, Agyemang NB Ashani Y, Murelli RP, Tavis JE, Antiviral Res. 2017, 144, 164–172.; - PMC - PubMed
- Long KR, Lomonosova E, Li W, Ponzar NL, Villa JA, Touchette E, Rapp S, Liley RM, Murelli RP, Grigoryan A, Buller RM, Wilson L, Bial J, Sagartz JE, Tavis JE, Antiviral Res. 2018, 149, 41–47.; - PMC - PubMed
- For seminal studies, see Hu Y, Cheng X, Cao F, Huang A, Tavis JE, Antiviral Res. 2013, 99, 221–229. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

