Humoral immune response to COVID-19 vaccination in diabetes is age-dependent but independent of type of diabetes and glycaemic control: The prospective COVAC-DM cohort study
- PMID: 34984802
- PMCID: PMC9303917
- DOI: 10.1111/dom.14643
Humoral immune response to COVID-19 vaccination in diabetes is age-dependent but independent of type of diabetes and glycaemic control: The prospective COVAC-DM cohort study
Abstract
Aims: To investigate the seroconversion following first and second COVID-19 vaccination in people with type 1 and type 2 diabetes in relation to glycaemic control prior to vaccination and to analyse the response in comparison to individuals without diabetes.
Materials and methods: This prospective, multicentre cohort study analysed people with type 1 and type 2 diabetes and a glycated haemoglobin level ≤58 mmol/mol (7.5%) or >58 mmol/mol (7.5%), respectively, and healthy controls. Roche's Elecsys anti-SARS-CoV-2 S immunoassay targeting the receptor-binding domain was used to quantify anti-spike protein antibodies 7 to 14 days after the first and 14 to 21 days after the second vaccination.
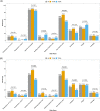
Results: A total of 86 healthy controls were enrolled in the study, as well as 161 participants with diabetes, of whom 150 (75 with type 1 diabetes and 75 with type 2 diabetes) were eligible for the analysis. After the first vaccination, only 52.7% of participants in the type 1 diabetes group and 48.0% of those in the type 2 diabetes group showed antibody levels above the cut-off for positivity. Antibody levels after the second vaccination were similar in participants with type 1 diabetes, participants with type 2 diabetes and healthy controls after adjusting for age, sex and multiple testing (P > 0.05). Age (r = -0.45, P < 0.001) and glomerular filtration rate (r = 0.28, P = 0.001) were significantly associated with antibody response.
Conclusions: Anti-SARS-CoV-2 S receptor-binding domain antibody levels after the second vaccination were comparable in healthy controls and in participants with type 1 and type 2 diabetes, irrespective of glycaemic control. Age and renal function correlated significantly with the extent of antibody levels.
Keywords: COVID-19; observational study; type 1 diabetes; type 2 diabetes.
© 2022 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
None of the authors have conflicts of interest to declare.
Figures


Similar articles
-
Early humoral response to COVID-19 vaccination in patients living with obesity and diabetes in France. The COVPOP OBEDIAB study with results from the ANRS0001S COV-POPART cohort.Metabolism. 2023 May;142:155412. doi: 10.1016/j.metabol.2023.155412. Epub 2023 Jan 31. Metabolism. 2023. PMID: 36731720 Free PMC article.
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
The persistence of anti-Spike antibodies following two SARS-CoV-2 vaccine doses in patients on immunosuppressive therapy compared to healthy controls-a prospective cohort study.BMC Med. 2022 Oct 5;20(1):378. doi: 10.1186/s12916-022-02587-8. BMC Med. 2022. PMID: 36199139 Free PMC article.
-
BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers.Lancet Respir Med. 2021 Sep;9(9):999-1009. doi: 10.1016/S2213-2600(21)00220-4. Epub 2021 Jul 2. Lancet Respir Med. 2021. PMID: 34224675 Free PMC article.
-
Severe acute respiratory syndrome coronavirus 2 spike antibody level decline is more pronounced after the second vaccination, but response to the third vaccination is similar in people with type 1 and type 2 diabetes compared with healthy controls: The prospective COVAC-DM cohort study.Diabetes Obes Metab. 2023 Jan;25(1):314-318. doi: 10.1111/dom.14855. Epub 2022 Sep 15. Diabetes Obes Metab. 2023. PMID: 36057945 Free PMC article. No abstract available.
Cited by
-
Correlation between COVID-19 vaccination and diabetes mellitus: A systematic review.World J Diabetes. 2023 Jun 15;14(6):892-918. doi: 10.4239/wjd.v14.i6.892. World J Diabetes. 2023. PMID: 37383586 Free PMC article.
-
Sulfonohydrazide as a potential inhibitor of SARS-CoV-2 infection.Sci Rep. 2025 May 28;15(1):18732. doi: 10.1038/s41598-025-03685-2. Sci Rep. 2025. PMID: 40437072 Free PMC article.
-
Comparing the B and T cell-mediated immune responses in patients with type 2 diabetes receiving mRNA or inactivated COVID-19 vaccines.Front Immunol. 2022 Oct 11;13:1018393. doi: 10.3389/fimmu.2022.1018393. eCollection 2022. Front Immunol. 2022. PMID: 36304475 Free PMC article.
-
Immunogenicity of SARS-CoV-2 BNT162b2 Vaccine in People with Diabetes: A Prospective Observational Study.Vaccines (Basel). 2022 Mar 2;10(3):382. doi: 10.3390/vaccines10030382. Vaccines (Basel). 2022. PMID: 35335014 Free PMC article.
-
Comparative Analysis of Predictive Interstitial Glucose Level Classification Models.Sensors (Basel). 2023 Oct 6;23(19):8269. doi: 10.3390/s23198269. Sensors (Basel). 2023. PMID: 37837098 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

