Prospects in the management of patients with follicular lymphoma beyond first-line therapy
- PMID: 34985231
- PMCID: PMC8719064
- DOI: 10.3324/haematol.2021.278717
Prospects in the management of patients with follicular lymphoma beyond first-line therapy
Abstract
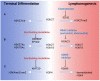
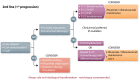
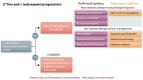
The management of patients with relapsed or refractory follicular lymphoma has evolved markedly in the last decade, with the availability of new classes of agents (phosphoinositide 3-kinase inhibitors, immunomodulators, epigenetic therapies, and chimeric antigen receptor T cells) supplementing the multiple approaches already available (cytotoxic agents, anti-CD20 antibodies, radiation therapy, radioimmunotherapy, and autologous and allogeneic transplants). The diversity of clinical scenarios, the flood of data derived from phase II studies, and the lack of randomized studies comparing treatment strategies preclude firm recommendations and require personalized decisions. Patients with early progression require specific attention given the risk of histological transformation and their lower response to standard therapies. In sequencing therapies, one must consider prior treatment regimens and the potential need for future lines of therapy. Careful evaluation of risks and expected benefits of available options, which vary depending on location and socioeconomics, should be undertaken, and should incorporate the patient's goals. Preserving quality of life for these patients is essential, given the likelihood of years to decades of survival and the possibility of multiple lines of therapy. The current landscape is likely to continue evolving rapidly with other effective agents emerging (notably bispecific antibodies and other targeted therapies), and multiple combinations being evaluated. It is hoped that new treatments under development will achieve longer progression-free intervals and minimize toxicity. A better understanding of disease biology and the mechanisms of these different agents should provide further insights to select the optimal therapy at each stage of disease.
Figures

References
-
- Junlén H, Peterson S, Kimby E, et al. Follicular lymphoma in Sweden: nationwide improved survival in the rituximab era, particularly in elderly women: a Swedish Lymphoma Registry study. Leukemia. 2015;29(3):668-676. - PubMed
-
- Al-Tourah AJ, Gill KK, Chhanabhai M, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(32):5165-5169. - PubMed
-
- Federico M, Barrigón MDC, Marcheselli L, et al. Rituximab and the risk of transformation of follicular lymphoma: a retrospective pooled analysis. Lancet Haematol. 2018;5(8):e359-367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

