Assembly of Bleomycin Saccharide-Decorated Spherical Nucleic Acids
- PMID: 34985282
- PMCID: PMC8778632
- DOI: 10.1021/acs.bioconjchem.1c00539
Assembly of Bleomycin Saccharide-Decorated Spherical Nucleic Acids
Abstract
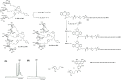
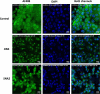
Glyco-decorated spherical nucleic acids (SNAs) may be attractive delivery vehicles, emphasizing the sugar-specific effect on the outer sphere of the construct and at the same time hiding unfavorable distribution properties of the loaded oligonucleotides. As examples of such nanoparticles, tripodal sugar constituents of bleomycin were synthesized and conjugated with a fluorescence-labeled antisense oligonucleotide (AONARV7). Successive copper(I)-catalyzed azide-alkyne and strain-promoted alkyne-nitrone cycloadditions (SPANC) were utilized for the synthesis. Then, the glyco-AONARV7 conjugates were hybridized with complementary strands of a C60-based molecular spherical nucleic acid (i.e., a hybridization-mediated carrier). The formation and stability of these assembled glyco-decorated SNAs were evaluated by polyacrylamide gel electrophoresis (PAGE), UV melting profile analysis, and time-resolved fluorescence spectroscopy. Association constants were extracted from time-resolved fluorescence data. Preliminary cellular uptake experiments of the glyco-AONARV7 conjugates (120 nM solutions) and of the corresponding glyco-decorated SNAs (10 nM solutions) with human prostate cancer cells (PC3) showed an efficient uptake in each case. A marked variation in intracellular distribution was observed.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Zheng D.; Giljohann D. A.; Chen D. L.; Massich M. D.; Wang X. Q.; Iordanov H.; Mirkin C. A.; Paller A. S. Topical Delivery of SiRNA-Based Spherical Nucleic Acid Nanoparticle Conjugates for Gene Regulation. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 11975–11980. 10.1073/pnas.1118425109. - DOI - PMC - PubMed
-
- Jensen S. A.; Day E. S.; Ko C. H.; Hurley L. A.; Luciano J. P.; Kouri F. M.; Merkel T. J.; Luthi A. C.; Patel P. C.; et al. Spherical Nucleic Acid Nanoparticle Conjugates as an RNAi-Based Therapy for Glioblastoma. Sci. Transl. Med. 2013, 5, 209ra152-209ra152 10.1126/scitranslmed.3006839. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

