Opportunities and challenges of applying advanced X-ray spectroscopy to actinide and lanthanide N-donor ligand systems
- PMID: 34985423
- PMCID: PMC8733980
- DOI: 10.1107/S1600577521012091
Opportunities and challenges of applying advanced X-ray spectroscopy to actinide and lanthanide N-donor ligand systems
Abstract
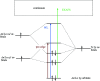
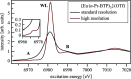
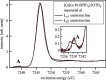
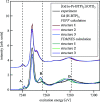
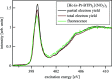
N-donor ligands such as n-Pr-BTP [2,6-bis(5,6-dipropyl-1,2,4-triazin-3-yl)pyridine] preferentially bind trivalent actinides (An3+) over trivalent lanthanides (Ln3+) in liquid-liquid separation. However, the chemical and physical processes responsible for this selectivity are not yet well understood. Here, an explorative comparative X-ray spectroscopy and computational (L3-edge) study for the An/Ln L3-edge and the N K-edge of [An/Ln(n-Pr-BTP)3](NO3)3, [Ln(n-Pr-BTP)3](CF3SO3)3 and [Ln(n-Pr-BTP)3](ClO4)3 complexes is presented. High-resolution X-ray absorption near-edge structure (HR-XANES) L3-edge data reveal additional features in the pre- and post-edge range of the spectra that are investigated using the quantum chemical codes FEFF and FDMNES. X-ray Raman spectroscopy studies demonstrate the applicability of this novel technique for investigations of liquid samples of partitioning systems at the N K-edge.
Keywords: CC-RIXS; HR-XANES; X-ray Raman; actinides; lanthanides; partitioning.
open access.
Figures
















References
-
- Asakura, H., Shishido, T., Fuchi, S., Teramura, K. & Tanaka, T. (2014). J. Phys. Chem. C, 118, 20881–20888.
-
- Asakura, H., Shishido, T., Teramura, K. & Tanaka, T. (2015). J. Phys. Chem. C, 119, 8070–8077.
-
- Banik, N. L., Schimmelpfennig, B., Marquardt, C. M., Brendebach, B., Geist, A. & Denecke, M. A. (2010). Dalton Trans. 39, 5117–5122. - PubMed
-
- Bartolomé, F., Krisch, M. H., Raoux, D. & Tonnerre, J.-M. (1999). Phys. Rev. B, 60, 13497–13506.
-
- Bartolomé, F., Tonnerre, J. M., Sève, L., Raoux, D., Chaboy, J., García, L. M., Krisch, M. & Kao, C. C. (1997). Phys. Rev. Lett. 79, 3775–3778.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

