Microbial Signatures in The Rodent Eyes With Retinal Dysfunction and Diabetic Retinopathy
- PMID: 34985498
- PMCID: PMC8742510
- DOI: 10.1167/iovs.63.1.5
Microbial Signatures in The Rodent Eyes With Retinal Dysfunction and Diabetic Retinopathy
Abstract
Purpose: The gut microbiome has been linked to disease pathogenesis through their interaction in metabolic, endocrine, and immune functions. The goal of this study was to determine whether the gut and plasma microbiota could transfer microbes to the retina in type 1 diabetic mice with retinopathy.
Methods: We analyzed the fecal, plasma, whole globe, and retina microbiome in Akita mice and compared with age-matched wild-type (WT) mice using 16S rRNA sequencing and metatranscriptomic analysis. To eliminate the contribution of the ocular surface and plasma microbiome, mice were perfused with sterile saline solution, the whole globes were extracted, and the neural retina was removed under sterile conditions for retinal microbiome.
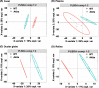
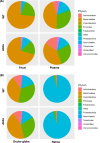
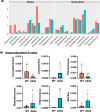
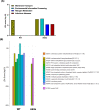
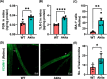
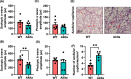
Results: Our microbiome analysis revealed that Akita mice demonstrated a distinct pattern of microbes within each source: feces, plasma, whole globes, and retina. WT mice and Akita mice experienced transient bacteremia in the plasma and retina. Bacteria were identified in the retina of the Akita mice, specifically Corynebacterium, Pseudomonas, Lactobacillus, Staphylococcus, Enterococcus, and Bacillus. Significantly increased levels of peptidoglycan (0.036 ± 0.001 vs. 0.023 ± 0.002; P < 0.002) and TLR2 (3.47 ± 0.15 vs. 1.99 ± 0.07; P < 0.0001) were observed in the retina of Akita mice compared to WT. Increased IBA+ cells in the retina, reduced a- and b-waves on electroretinography, and increased acellular capillary formation demonstrated the presence of retinopathy in the Akita cohort compared to WT mice.
Conclusions: Together, our findings suggest that transient bacteremia exists in the plasma and retina of both cohorts. The bacteria found in Akita mice are distinct from WT mice and may contribute to development of retinal inflammation and barrier dysfunction in retinopathy.
Conflict of interest statement
Disclosure:
Figures

Similar articles
-
Sustained ACE2 Expression by Probiotic Improves Integrity of Intestinal Lymphatics and Retinopathy in Type 1 Diabetic Model.J Clin Med. 2023 Feb 23;12(5):1771. doi: 10.3390/jcm12051771. J Clin Med. 2023. PMID: 36902558 Free PMC article.
-
Changes in the gut microbiota and derived fecal metabolites may play a role in tacrolimus-induced diabetes in mice.Future Microbiol. 2025 Feb;20(3):237-246. doi: 10.1080/17460913.2024.2444761. Epub 2024 Dec 22. Future Microbiol. 2025. PMID: 39711145
-
Early life bifidobacterial mother-infant transmission: greater contribution from the infant gut to human milk revealed by microbiomic and culture-based methods.mSystems. 2025 Jul 22;10(7):e0048025. doi: 10.1128/msystems.00480-25. Epub 2025 Jun 25. mSystems. 2025. PMID: 40558046 Free PMC article.
-
Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy.Cochrane Database Syst Rev. 2015 Jan 7;1(1):CD008081. doi: 10.1002/14651858.CD008081.pub3. Cochrane Database Syst Rev. 2015. PMID: 25564068 Free PMC article.
-
Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD008081. doi: 10.1002/14651858.CD008081.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 Jan 07;1:CD008081. doi: 10.1002/14651858.CD008081.pub3. PMID: 21735421 Updated.
Cited by
-
The ideal treatment timing for diabetic retinopathy: the molecular pathological mechanisms underlying early-stage diabetic retinopathy are a matter of concern.Front Endocrinol (Lausanne). 2023 Nov 9;14:1270145. doi: 10.3389/fendo.2023.1270145. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38027131 Free PMC article. Review.
-
Sodium butyrate ameliorates diabetic retinopathy in mice via the regulation of gut microbiota and related short-chain fatty acids.J Transl Med. 2023 Jul 7;21(1):451. doi: 10.1186/s12967-023-04259-4. J Transl Med. 2023. PMID: 37420234 Free PMC article.
-
Sustained ACE2 Expression by Probiotic Improves Integrity of Intestinal Lymphatics and Retinopathy in Type 1 Diabetic Model.J Clin Med. 2023 Feb 23;12(5):1771. doi: 10.3390/jcm12051771. J Clin Med. 2023. PMID: 36902558 Free PMC article.
-
The gut microbiota regulates diabetic retinopathy in adult rats.Front Microbiol. 2025 Jan 29;16:1479792. doi: 10.3389/fmicb.2025.1479792. eCollection 2025. Front Microbiol. 2025. PMID: 39949626 Free PMC article.
-
Immune Fingerprint in Diabetes: Ocular Surface and Retinal Inflammation.Int J Mol Sci. 2023 Jun 6;24(12):9821. doi: 10.3390/ijms24129821. Int J Mol Sci. 2023. PMID: 37372968 Free PMC article. Review.
References
-
- Baquero F, Nombela C.. The microbiome as a human organ. Clin Microbiol Infect. 2012; 18(Suppl 4): 2–4. - PubMed
-
- Price PB. The Bacteriology of Normal Skin; A New Quantitative Test Applied to a Study of the Bacterial Flora and the Disinfectant Action of Mechanical Cleansing. The Journal of Infectious Diseases. 1938; 63: 301–318.
-
- Sekirov I, Russell SL, Antunes LC, Finlay BB.. Gut microbiota in health and disease. Physiol Rev. 2010; 90: 859–904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

