Pathways Toward a Functional HIV-1 Cure: Balancing Promise and Perils of CRISPR Therapy
- PMID: 34985679
- PMCID: PMC9262118
- DOI: 10.1007/978-1-0716-1871-4_27
Pathways Toward a Functional HIV-1 Cure: Balancing Promise and Perils of CRISPR Therapy
Abstract
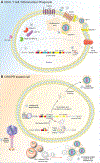
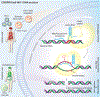
First identified as a viral defense mechanism, clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated proteins (Cas) has been transformed into a gene-editing tool. It now affords promise in the treatment and potential eradication of a range of divergent genetic, cancer, infectious, and degenerative diseases. Adapting CRISPR-Cas into a programmable endonuclease directed guide RNA (gRNA) has attracted international attention. It was recently awarded the 2020 Nobel Prize in Chemistry. The limitations of this technology have also been identified and work has been made in providing potential remedies. For treatment of the human immunodeficiency virus type one (HIV-1), in particular, a CRISPR-Cas9 approach was adapted to target then eliminate latent proviral DNA. To this end, we reviewed the promise and perils of CRISPR-Cas gene-editing strategies for HIV-1 elimination. Obstacles include precise delivery to reservoir tissue and cell sites of latent HIV-1 as well as assay sensitivity and specificity. The detection and consequent excision of common viral strain sequences and the avoidance of off-target activity will serve to facilitate a final goal of HIV-1 DNA elimination and accelerate testing in infected animals ultimately for use in man.
Keywords: CRISPR-Cas; CRISPR-associated proteins; RNA.
© 2022. Springer Science+Business Media, LLC, part of Springer Nature.
Figures
Similar articles
-
Gene Therapy with CRISPR/Cas9 Coming to Age for HIV Cure.AIDS Rev. 2017 Oct-Dec;19(3):167-172. AIDS Rev. 2017. PMID: 29019352
-
CRISPR-Cas9 Mediated Exonic Disruption for HIV-1 Elimination.EBioMedicine. 2021 Nov;73:103678. doi: 10.1016/j.ebiom.2021.103678. Epub 2021 Nov 10. EBioMedicine. 2021. PMID: 34774454 Free PMC article.
-
CRISPR/Cas9 Ablation of Integrated HIV-1 Accumulates Proviral DNA Circles with Reformed Long Terminal Repeats.J Virol. 2021 Nov 9;95(23):e0135821. doi: 10.1128/JVI.01358-21. Epub 2021 Sep 22. J Virol. 2021. PMID: 34549986 Free PMC article.
-
CRISPR-Cas based antiviral strategies against HIV-1.Virus Res. 2018 Jan 15;244:321-332. doi: 10.1016/j.virusres.2017.07.020. Epub 2017 Jul 29. Virus Res. 2018. PMID: 28760348 Review.
-
Clustered regularly interspaced palindromic repeats-cas9-based strategies towards HIV eradication: A literature review.J Pak Med Assoc. 2021 Feb;71(Suppl 2)(2):S134-S139. J Pak Med Assoc. 2021. PMID: 33785958 Review.
Cited by
-
Neuroinflammation, Blood-Brain Barrier, and HIV Reservoirs in the CNS: An In-Depth Exploration of Latency Mechanisms and Emerging Therapeutic Strategies.Viruses. 2025 Apr 16;17(4):572. doi: 10.3390/v17040572. Viruses. 2025. PMID: 40285014 Free PMC article. Review.
-
Targeted shock-and-kill HIV-1 gene therapy approach combining CRISPR activation, suicide gene tBid and retargeted adenovirus delivery.Gene Ther. 2024 Mar;31(3-4):74-84. doi: 10.1038/s41434-023-00413-1. Epub 2023 Aug 9. Gene Ther. 2024. PMID: 37558852 Free PMC article.
References
-
- Cohen J CRISPR, the revolutionary genetic ‘scissors,’ honored by Chemistry Nobel. 2020 (2020). <https://www.sciencemag.org/news/2020/10/crispr-revolutionary-genetic-sci...>.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

