Preoperative and Postoperative Systemic Therapy for Operable Non-Small-Cell Lung Cancer
- PMID: 34985966
- PMCID: PMC8853628
- DOI: 10.1200/JCO.21.01589
Preoperative and Postoperative Systemic Therapy for Operable Non-Small-Cell Lung Cancer
Abstract
Cisplatin-based adjuvant chemotherapy remains the standard of care for patients with resected stage II or III non-small-cell lung cancer. However, biomarker-informed clinical trials are starting to push the management of early-stage lung cancer beyond cytotoxic chemotherapy. This review explores recent and ongoing studies focused on improving cytotoxic chemotherapy and incorporating targeted and immunotherapies in the management of early-stage, resectable lung cancer. Adjuvant osimertinib for patients with EGFR-mutant tumors, preoperative chemoimmunotherapy, and adjuvant immunotherapy could improve outcomes for selected patients with resectable lung cancer, and ongoing or planned studies leveraging biomarkers, immunotherapy, and targeted therapy may further improve survival. We also discuss the unique barriers associated with clinical trials of early-stage lung cancer and the need for innovative trial designs to overcome these challenges.
Conflict of interest statement
Figures
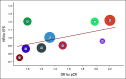
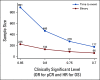
References
-
- Goldstraw P, Chansky K, Crowley J, et al. : The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 11:39-51, 2016 - PubMed
-
- Pignon JP, Tribodet H, Scagliotti GV, et al. : Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol 26:3552-3559, 2008 - PubMed
-
- Strauss GM, Herndon JE II, Maddaus MA, et al. : Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 26:5043-5051, 2008 - PMC - PubMed
-
- National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Non-Small Cell Lung Cancer, 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

