A Pseudorabies Virus Serine/Threonine Kinase, US3, Promotes Retrograde Transport in Axons via Akt/mToRC1
- PMID: 34985995
- PMCID: PMC8906396
- DOI: 10.1128/JVI.01752-21
A Pseudorabies Virus Serine/Threonine Kinase, US3, Promotes Retrograde Transport in Axons via Akt/mToRC1
Abstract
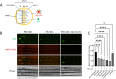
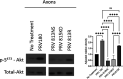
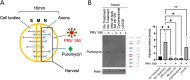
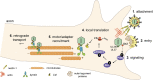
Infection of peripheral axons by alpha herpesviruses (AHVs) is a critical stage in establishing a lifelong infection in the host. Upon entering the cytoplasm of axons, AHV nucleocapsids and associated inner-tegument proteins must engage the cellular retrograde transport machinery to promote the long-distance movement of virion components to the nucleus. The current model outlining this process is incomplete, and further investigation is required to discover all viral and cellular determinants involved as well as the temporality of the events. Using a modified trichamber system, we have discovered a novel role of the pseudorabies virus (PRV) serine/threonine kinase US3 in promoting efficient retrograde transport of nucleocapsids. We discovered that transporting nucleocapsids move at similar velocities in both the presence and absence of a functional US3 kinase; however, fewer nucleocapsids are moving when US3 is absent, and they move for shorter periods of time before stopping, suggesting that US3 is required for efficient nucleocapsid engagement with the retrograde transport machinery. This led to fewer nucleocapsids reaching the cell bodies to produce a productive infection 12 h later. Furthermore, US3 was responsible for the induction of local translation in axons as early as 1 h postinfection (hpi) through the stimulation of a phosphatidylinositol 3-kinase (PI3K)/Akt-mToRC1 pathway. These data describe a novel role for US3 in the induction of local translation in axons during AHV infection, a critical step in transport of nucleocapsids to the cell body. IMPORTANCE Neurons are highly polarized cells with axons that can reach centimeters in length. Communication between axons at the periphery and the distant cell body is a relatively slow process involving the active transport of chemical messengers. There is a need for axons to respond rapidly to extracellular stimuli. Translation of repressed mRNAs present within the axon occurs to enable rapid, localized responses independently of the cell body. AHVs have evolved a way to hijack local translation in the axons to promote their transport to the nucleus. We have determined the cellular mechanism and viral components involved in the induction of axonal translation. The US3 serine/threonine kinase of PRV activates Akt-mToRC1 signaling pathways early during infection to promote axonal translation. When US3 is not present, the number of moving nucleocapsids and their processivity are reduced, suggesting that US3 activity is required for efficient engagement of nucleocapsids with the retrograde transport machinery.
Keywords: Akt; PRV; US3; axon; intracellular transport; kinase; mToRC1; pseudorabies virus; retrograde transport; translation; viral entry.
Conflict of interest statement
The authors declare no conflict of interest.
We declare that we have no competing interests.
Figures