Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma
- PMID: 34986285
- PMCID: PMC9844513
- DOI: 10.1056/NEJMoa2109970
Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma
Abstract
Background: Lymphocyte-activation gene 3 (LAG-3) and programmed death 1 (PD-1) are distinct inhibitory immune checkpoints that contribute to T-cell exhaustion. The combination of relatlimab, a LAG-3-blocking antibody, and nivolumab, a PD-1-blocking antibody, has been shown to be safe and to have antitumor activity in patients with previously treated melanoma, but the safety and activity in patients with previously untreated melanoma need investigation.
Methods: In this phase 2-3, global, double-blind, randomized trial, we evaluated relatlimab and nivolumab as a fixed-dose combination as compared with nivolumab alone when administered intravenously every 4 weeks to patients with previously untreated metastatic or unresectable melanoma. The primary end point was progression-free survival as assessed by blinded independent central review.
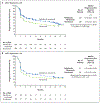
Results: The median progression-free survival was 10.1 months (95% confidence interval [CI], 6.4 to 15.7) with relatlimab-nivolumab as compared with 4.6 months (95% CI, 3.4 to 5.6) with nivolumab (hazard ratio for progression or death, 0.75 [95% CI, 0.62 to 0.92]; P = 0.006 by the log-rank test). Progression-free survival at 12 months was 47.7% (95% CI, 41.8 to 53.2) with relatlimab-nivolumab as compared with 36.0% (95% CI, 30.5 to 41.6) with nivolumab. Progression-free survival across key subgroups favored relatlimab-nivolumab over nivolumab. Grade 3 or 4 treatment-related adverse events occurred in 18.9% of patients in the relatlimab-nivolumab group and in 9.7% of patients in the nivolumab group.
Conclusions: The inhibition of two immune checkpoints, LAG-3 and PD-1, provided a greater benefit with regard to progression-free survival than inhibition of PD-1 alone in patients with previously untreated metastatic or unresectable melanoma. Relatlimab and nivolumab in combination showed no new safety signals. (Funded by Bristol Myers Squibb; RELATIVITY-047 ClinicalTrials.gov number, NCT03470922.).
Copyright © 2022 Massachusetts Medical Society.
Figures


Comment in
-
A New Combination Immunotherapy in Advanced Melanoma.N Engl J Med. 2022 Jan 6;386(1):91-92. doi: 10.1056/NEJMe2116892. N Engl J Med. 2022. PMID: 34986291 No abstract available.
-
LAG3 inhibition improves outcomes.Nat Rev Clin Oncol. 2022 Mar;19(3):149. doi: 10.1038/s41571-022-00602-8. Nat Rev Clin Oncol. 2022. PMID: 35031714 No abstract available.
-
CTLA-4 Blockade Resistance after Relatlimab and Nivolumab.N Engl J Med. 2022 Apr 28;386(17):1668-1669. doi: 10.1056/NEJMc2119768. N Engl J Med. 2022. PMID: 35476655 No abstract available.
-
Nivolumab with or without Relatlimab in Untreated Advanced Melanoma.N Engl J Med. 2022 May 12;386(19):1860. doi: 10.1056/NEJMc2201558. N Engl J Med. 2022. PMID: 35544393 No abstract available.
-
Relatlimab and nivolumab in the treatment of melanoma.Cell. 2022 Dec 22;185(26):4866-4869. doi: 10.1016/j.cell.2022.12.003. Cell. 2022. PMID: 36563660
References
-
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535–46. - PubMed
-
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J Clin Oncol 2021;39:Suppl 15: 9506. abstract.
-
- Workman CJ, Cauley LS, Kim I-J, Blackman MA, Woodland DL, Vignali DAA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol 2004;172:5450–5. - PubMed
-
- Hemon P, Jean-Louis F, Ramgolam K, et al. MHC class II engagement by its ligand LAG-3 (CD223) contributes to melanoma resistance to apoptosis. J Immunol 2011;186:5173–83. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
