Distinct Dibasic Cleavage Specificities of Neuropeptide-Producing Cathepsin L and Cathepsin V Cysteine Proteases Compared to PC1/3 and PC2 Serine Proteases
- PMID: 34986304
- PMCID: PMC9070308
- DOI: 10.1021/acschemneuro.1c00653
Distinct Dibasic Cleavage Specificities of Neuropeptide-Producing Cathepsin L and Cathepsin V Cysteine Proteases Compared to PC1/3 and PC2 Serine Proteases
Abstract
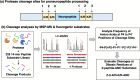
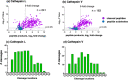
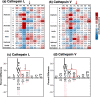
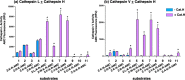
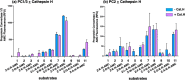
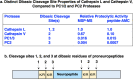
Neuropeptides, functioning as peptide neurotransmitters and hormones, are generated from proneuropeptide precursors by proteolytic processing at dibasic residue sites (i.e., KR, RK, KK, RR). The cysteine proteases cathepsin L and cathepsin V, combined with the serine proteases proprotein convertases 1 and 2 (PC1/3 and PC2), participate in proneuropeptide processing to generate active neuropeptides. To compare the dibasic cleavage properties of these proteases, this study conducted global, unbiased substrate profiling of these processing proteases using a diverse peptide library in multiplex substrate profiling by mass spectrometry (MSP-MS) assays. MSP-MS utilizes a library of 228 14-mer peptides designed to contain all possible protease cleavage sites, including the dibasic residue sites of KR, RK, KK, and RR. The comprehensive MSP-MS analyses demonstrated that cathepsin L and cathepsin V cleave at the N-terminal side and between the dibasic residues (e.g., ↓K↓R, ↓R↓K, and K↓K), with a preference for hydrophobic residues at the P2 position of the cleavage site. In contrast, the serine proteases PC1/3 and PC2 displayed cleavage at the C-terminal side of dibasic residues of a few peptide substrates. Further analyses with a series of dipeptide-AMC and tripeptide-AMC substrates containing variant dibasic sites with hydrophobic P2 residues indicated the preferences of cathepsin L and cathepsin V to cleave between dibasic residue sites with preferences for flanking hydrophobic residues at the P2 position consisting of Leu, Trp, Phe, and Tyr. Such hydrophobic amino acids reside in numerous proneuropeptides such as pro-NPY and proenkephalin that are known to be processed by cathepsin L. Notably, cathepsin L displayed the highest specific activity that was 10-, 64-, and 1268-fold greater than cathepsin V, PC1/3, and PC2, respectively. Peptide-AMC substrates with dibasic residues confirmed that PC1/3 and P2 cleaved almost exclusively at the C-terminal side of dibasic residues. These data demonstrate distinct dibasic cleavage site properties and a broad range of proteolytic activities of cathepsin L and cathepsin V, compared to PC1/3 and PC2, which participate in producing neuropeptides for cell-cell communication.
Keywords: cathepsin; mass spectrometry; neuropeptide; peptidomics; proprotein convertase; protease.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Felig P.; Baxter J. D.; Frohman L. A.. Endocrinology and Metabolism, 3rd ed.; McGraw Hill, Inc.: New York, 1981.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

