Glutamate in primary afferents is required for itch transmission
- PMID: 34986325
- PMCID: PMC8898340
- DOI: 10.1016/j.neuron.2021.12.007
Glutamate in primary afferents is required for itch transmission
Abstract
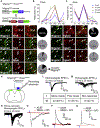
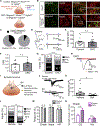
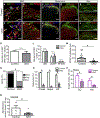
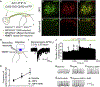
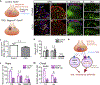
Whether glutamate or itch-selective neurotransmitters are used to confer itch specificity is still under debate. We focused on an itch-selective population of primary afferents expressing MRGPRA3, which highly expresses Vglut2 and the neuropeptide neuromedin B (Nmb), to investigate this question. Optogenetic stimulation of MRGPRA3+ afferents triggers scratching and other itch-related avoidance behaviors. Using a combination of optogenetics, spinal cord slice recordings, Vglut2 conditional knockout mice, and behavior assays, we showed that glutamate is essential for MRGPRA3+ afferents to transmit itch. We further demonstrated that MRGPRA3+ afferents form monosynaptic connections with both NMBR+ and NMBR- neurons and that NMB and glutamate together can enhance the activity of NMBR+ spinal DH neurons. Moreover, Nmb in MRGPRA3+ afferents and NMBR+ DH neurons are required for chloroquine-induced scratching. Together, our results establish a new model in which glutamate is an essential neurotransmitter in primary afferents for itch transmission, whereas NMB signaling enhances its activities.
Keywords: MRGPRA3+ afferents; NMB; VGLUT2; behavior assays; glutamate; high-speed imaging; itch-selective neurotransmitter; optogenetic stimulation; spinal cord slice recordings.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests V.G. is a member of the Neuron Advisory Board. V.G. is also a cofounder and board member of Capsida Biotherapeutics, a fully integrated AAV engineering and gene therapy company. The other authors declare no competing interests.
Figures
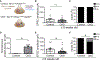

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

