Calcium-dependent ESCRT recruitment and lysosome exocytosis maintain epithelial integrity during Candida albicans invasion
- PMID: 34986345
- PMCID: PMC8755444
- DOI: 10.1016/j.celrep.2021.110187
Calcium-dependent ESCRT recruitment and lysosome exocytosis maintain epithelial integrity during Candida albicans invasion
Abstract
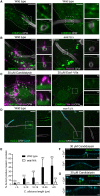
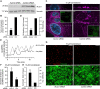
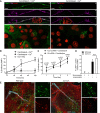
Candida albicans is both a commensal and an opportunistic fungal pathogen. Invading hyphae of C. albicans secrete candidalysin, a pore-forming peptide toxin. To prevent cell death, epithelial cells must protect themselves from direct damage induced by candidalysin and by the mechanical forces exerted by expanding hyphae. We identify two key Ca2+-dependent repair mechanisms employed by epithelial cells to withstand candidalysin-producing hyphae. Using camelid nanobodies, we demonstrate candidalysin secretion directly into the invasion pockets induced by elongating C. albicans hyphae. The toxin induces oscillatory increases in cytosolic [Ca2+], which cause hydrolysis of PtdIns(4,5)P2 and loss of cortical actin. Epithelial cells dispose of damaged membrane regions containing candidalysin by an Alg-2/Alix/ESCRT-III-dependent blebbing process. At later stages, plasmalemmal tears induced mechanically by invading hyphae are repaired by exocytic insertion of lysosomal membranes. These two repair mechanisms maintain epithelial integrity and prevent mucosal damage during both commensal growth and infection by C. albicans.
Keywords: ALG-2; Candida albicans; ESCRT; calcium; candidalysin; epithelia; lysosome; membrane damage; plasma membrane; repair.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Babiychuk E.B., Monastyrskaya K., Potez S., Draeger A. Intracellular Ca(2+) operates a switch between repair and lysis of streptolysin O-perforated cells. Cell Death Differ. 2009;16:1126–1134. - PubMed
-
- Baracca A., Sgarbi G., Solaini G., Lenaz G. Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F(0) during ATP synthesis. Biochim. Biophys. Acta. 2003;1606:137–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

