Trained immunity induction by the inactivated mucosal vaccine MV130 protects against experimental viral respiratory infections
- PMID: 34986349
- PMCID: PMC8755442
- DOI: 10.1016/j.celrep.2021.110184
Trained immunity induction by the inactivated mucosal vaccine MV130 protects against experimental viral respiratory infections
Abstract
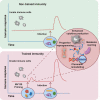
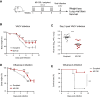
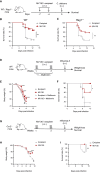
MV130 is an inactivated polybacterial mucosal vaccine that confers protection to patients against recurrent respiratory infections, including those of viral etiology. However, its mechanism of action remains poorly understood. Here, we find that intranasal prophylaxis with MV130 modulates the lung immune landscape and provides long-term heterologous protection against viral respiratory infections in mice. Intranasal administration of MV130 provides protection against systemic candidiasis in wild-type and Rag1-deficient mice lacking functional lymphocytes, indicative of innate immune-mediated protection. Moreover, pharmacological inhibition of trained immunity with metformin abrogates the protection conferred by MV130 against influenza A virus respiratory infection. MV130 induces reprogramming of both mouse bone marrow progenitor cells and in vitro human monocytes, promoting an enhanced cytokine production that relies on a metabolic shift. Our results unveil that the mucosal administration of a fully inactivated bacterial vaccine provides protection against viral infections by a mechanism associated with the induction of trained immunity.
Keywords: Candida albicans; human monocytes; influenza; macrophages; mucosal; polybacterial immunomodulator; respiratory infection; trained immunity; vaccine; viral infection.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.L.S., M.C., L.C., S. M.-C., and P.S.-L. were employees of Inmunotek S.L. at the time of the work. The D.S. lab receives funds from a collaboration agreement between CNIC and Inmunotek. The other authors declare no competing interests.
Figures


References
-
- Alecsandru D., Valor L., Sánchez-Ramón S., Gil J., Carbone J., Navarro J., Rodríguez J., Rodríguez-Sainz C., Fernández-Cruz E. Sublingual therapeutic immunization with a polyvalent bacterial preparation in patients with recurrent respiratory infections: immunomodulatory effect on antigen-specific memory CD4+ T cells and impact on clinical outcome. Clin. Exp. Immunol. 2011;164:100–107. - PMC - PubMed
-
- Arts R.J.W., Blok B.A., Aaby P., Joosten L.A.B., de Jong D., van der Meer J.W.M., Benn C.S., van Crevel R., Netea M.G. Long-term in vitro and in vivo effects of γ-irradiated BCG on innate and adaptive immunity. J. Leukoc. Biol. 2015;98:995–1001. - PubMed
-
- Arts R.J.W., Moorlag S.J.C.F.M., Novakovic B., Li Y., Wang S.Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B., et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100.e5. - PubMed
-
- Bekkering S., Quintin J., Joosten L.A.B., van der Meer J.W.M., Netea M.G., Riksen N.P. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 2014;34:1731–1738. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

