Augmentation strategies for treatment resistant major depression: A systematic review and network meta-analysis
- PMID: 34986373
- PMCID: PMC9328668
- DOI: 10.1016/j.jad.2021.12.134
Augmentation strategies for treatment resistant major depression: A systematic review and network meta-analysis
Abstract
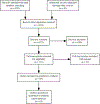
Objective: To compare the efficacy and discontinuation of augmentation agents in adult patients with treatment-resistant depression (TRD). We conducted a systematic review and network meta-analyses (NMA) to combine direct and indirect comparisons of augmentation agents.
Methods: We included randomized controlled trials comparing one active drug with another or with placebo following a treatment course up to 24 weeks. Nineteen agents were included: stimulants, atypical antipsychotics, thyroid hormones, antidepressants, and mood stabilizers. Data for response/remission and all-cause discontinuation rates were analyzed. We estimated effect-size by relative risk using pairwise and NMA with random-effects model.
Results: A total of 65 studies (N = 12,415) with 19 augmentation agents were included in the NMA. Our findings from the NMA for response rates, compared to placebo, were significant for: liothyronine, nortriptyline, aripiprazole, brexpiprazole, quetiapine, lithium, modafinil, olanzapine (fluoxetine), cariprazine, and lisdexamfetamine. For remission rates, compared to placebo, were significant for: thyroid hormone(T4), aripiprazole, brexpiprazole, risperidone, quetiapine, and olanzapine (fluoxetine). Compared to placebo, ziprasidone, mirtazapine, and cariprazine had statistically significant higher discontinuation rates. Overall, 24% studies were rated as having low risk of bias (RoB), 63% had moderate RoB and 13% had high RoB.
Limitations: Heterogeneity in TRD definitions, variable trial duration and methodological clinical design of older studies and small number of trials per comparisons.
Conclusions: This NMA suggests a superiority of the regulatory approved adjunctive atypical antipsychotics, thyroid hormones, dopamine compounds (modafinil and lisdexamfetamine) and lithium. Acceptability was lower with ziprasidone, mirtazapine, and cariprazine. Further research and head-to-head studies should be considered to strengthen the best available options for TRD.
Keywords: Efficacy; Mood disorders; Network Meta-analysis; Treatment resistant; Unipolar depression.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
NA Nuñez is supported by a grant from the National Institute of General Medical Sciences of the National Institutes of Health under award number T32 GM008685. MA Frye reports grant support from Assurex Health, Mayo Foundation and intellectual property licensed to Chymia LLC. with rights to receive future royalties. B Singh reports grant support from Mayo Clinic. All other authors report no financial relationships with commercial interests. All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Abolfazli R, Hosseini M, Ghanizadeh A, et al. , 2011. Double-blind randomized parallel-group clinical trial of efficacy of the combination fluoxetine plus modafinil versus fluoxetine plus placebo in the treatment of major depression. Depress. Anxiety 28, 297–302. - PubMed
-
- Altshuler LL, Bauer M, Frye MA, et al. , 2001. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am. J. Psychiatry 158, 1617–1622. - PubMed
-
- Appelberg BG, Syvalahti EK, Koskinen TE, et al. , 2001. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J. Clin. Psychiatry 62, 448–452. - PubMed
-
- Barbee JG, Thompson TR, Jamhour NJ, et al. , 2011. A double-blind placebo-controlled trial of lamotrigine as an antidepressant augmentation agent in treatment-refractory unipolar depression. J. Clin. Psychiatry 72, 1405–1412. - PubMed
-
- Barbosa L, Berk M, Vorster M, 2003. A double-blind, randomized, placebo-controlled trial of augmentation with lamotrigine or placebo in patients concomitantly treated with fluoxetine for resistant major depressive episodes. J. Clin. Psychiatry 64, 403–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

