Pharmaceutical strategies for preventing toxicity and promoting antioxidant and anti-inflammatory actions of bilirubin
- PMID: 34986721
- PMCID: PMC8741241
- DOI: 10.1080/14756366.2021.2020773
Pharmaceutical strategies for preventing toxicity and promoting antioxidant and anti-inflammatory actions of bilirubin
Abstract
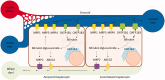
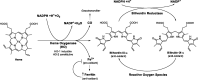
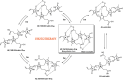
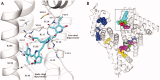
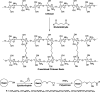
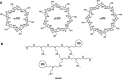
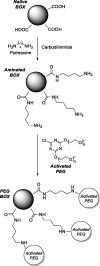
Bilirubin (BR) is the final product of haem catabolism. Disruptions along BR metabolic/transport pathways resulting from inherited disorders can increase plasma BR concentration (hyperbilirubinaemia). Unconjugated hyperbilirubinemia may induce BR accumulation in brain, potentially causing irreversible neurological damage, a condition known as BR encephalopathy or kernicterus, to which newborns are especially vulnerable. Numerous pharmaceutical strategies, mostly based on hemoperfusion, have been proposed over the last decades to identify new valid, low-risk alternatives for BR removal from plasma. On the other hand, accumulating evidence indicates that BR produces health benefits due to its potent antioxidant, anti-inflammatory and immunomodulatory action with a significant potential for the treatment of a multitude of diseases. The present manuscript reviews both such aspects of BR pharmacology, gathering literature data on applied pharmaceutical strategies adopted to: (i) reduce the plasma BR concentration for preventing neurotoxicity; (ii) produce a therapeutic effect based on BR efficacy in the treatment of many disorders.
Keywords: Bilirubin; antioxidant; hyperbilirubinaemia; jaundice.
Conflict of interest statement
CT Supuran is Editor-in-Chief of the Journal of Enzyme Inhibition and Medicinal Chemistry. He was not involved in the assessment, peer review, or decision-making process of this paper. The authors have no relevant affiliations of financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures
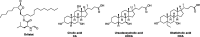


Similar articles
-
Bioadhesive hydrogel comprising bilirubin/β-cyclodextrin inclusion complexes promote diabetic wound healing.Pharm Biol. 2021 Dec;59(1):1139-1149. doi: 10.1080/13880209.2021.1964543. Pharm Biol. 2021. PMID: 34425063 Free PMC article.
-
Bilirubin and brain: A pharmacological approach.Neuropharmacology. 2017 May 15;118:113-123. doi: 10.1016/j.neuropharm.2017.03.013. Epub 2017 Mar 14. Neuropharmacology. 2017. PMID: 28315352 Review.
-
Contribution of inflammatory processes to nerve cell toxicity by bilirubin and efficacy of potential therapeutic agents.Curr Pharm Des. 2009;15(25):2915-26. doi: 10.2174/138161209789058165. Curr Pharm Des. 2009. PMID: 19754368 Review.
-
Easy Diagnosis of Jaundice: A Smartphone-Based Nanosensor Bioplatform Using Photoluminescent Bacterial Nanopaper for Point-of-Care Diagnosis of Hyperbilirubinemia.ACS Sens. 2019 Apr 26;4(4):1063-1071. doi: 10.1021/acssensors.9b00275. Epub 2019 Mar 29. ACS Sens. 2019. PMID: 30896150
-
Gene replacement therapy for genetic hepatocellular jaundice.Clin Rev Allergy Immunol. 2015 Jun;48(2-3):243-53. doi: 10.1007/s12016-014-8454-7. Clin Rev Allergy Immunol. 2015. PMID: 25315738 Review.
Cited by
-
Effects of bilirubin on the development and electrical activity of neural circuits.Front Cell Neurosci. 2023 Mar 21;17:1136250. doi: 10.3389/fncel.2023.1136250. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37025700 Free PMC article. Review.
-
Serum bilirubin levels in primary Sjögren's syndrome: an association with interstitial lung disease.BMC Pulm Med. 2023 Sep 30;23(1):366. doi: 10.1186/s12890-023-02672-5. BMC Pulm Med. 2023. Retraction in: BMC Pulm Med. 2024 Feb 7;24(1):73. doi: 10.1186/s12890-024-02876-3. PMID: 37777728 Free PMC article. Retracted.
-
Antioxidant Activity of Bilirubin in Micellar and Liposomal Systems Is pH-Dependent.Antioxidants (Basel). 2024 Mar 30;13(4):426. doi: 10.3390/antiox13040426. Antioxidants (Basel). 2024. PMID: 38671874 Free PMC article.
-
Relationship between bilirubin and systemic lupus erythematosus: A systematic review and meta-analysis.Immun Inflamm Dis. 2023 Dec;11(12):e1115. doi: 10.1002/iid3.1115. Immun Inflamm Dis. 2023. PMID: 38156396 Free PMC article.
-
Establishment of predictive models for postoperative delirium in elderly patients after knee/hip surgery based on total bilirubin concentration: machine learning algorithms.BMC Anesthesiol. 2025 Jul 30;25(1):375. doi: 10.1186/s12871-025-03259-9. BMC Anesthesiol. 2025. PMID: 40739627 Free PMC article.
References
-
- London IM, West R, Shemin D, Rittenberg D.. On the origin of bile pigment in normal man. J Biol Chem 1950;184:351–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
